Chitosan oligosaccharide enhances the anti-cancer effects of 5-fluorouracil on SNU-C5 colorectal cancer cells by activating ERK
- PMID: 40191714
- PMCID: PMC11964875
- DOI: 10.32604/or.2024.052003
Chitosan oligosaccharide enhances the anti-cancer effects of 5-fluorouracil on SNU-C5 colorectal cancer cells by activating ERK
Abstract
Background: Chitosan oligosaccharide (COS) is the major degradation product of chitosan by enzymatic processes. COS, with complete water solubility, exerts significant biological effects, including anti-cancer activity. We investigated the anti-tumor effects of COS on colorectal cancer as effective therapeutic methods with low side effects are lacking.
Methods: COS was obtained from low molecular weight chitosan by an enzymatic method and the anti-cancer effects were measured by cell viability assay, flow cytometry analysis, Western blotting, and xenograft.
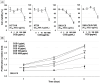
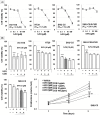
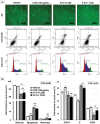
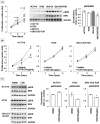
Results: COS suppressed the proliferation of SNU-C5 cells compared to other colorectal cancer cells, but higher concentrations were required in the xenograft model. Co-treatment with 5-fluorouracil (5-FU) and COS enhanced the anti-cancer effects of 5-FU in SNU-C5 cells in vitro and in vivo. Flow cytometry revealed that COS induced cell cycle arrest at the G0/G1 phase without 5-FU or at the S and G2/M phases with 5-FU but did not affect cell death pathways. COS increased extracellular signal-regulated protein kinase (ERK) activation with or without 5-FU, whereas 5-FU treatment increased p53 activation. A low-dose of an ERK inhibitor suppressed COS-induced ERK activation and resulted in higher proliferation compared with COS.
Conclusions: These results suggest that COS might enhance the anti-cancer effects of 5-FU in SNU-C5 colorectal cancer cells by activating ERK.
Keywords: 5-Fluorouracil (5-FU); Chitosan oligosaccharide (COS); Colorectal cancer (CRC); ERK (Extracellular signal-regulated protein kinase).
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest to report regarding the present study.
Figures





Similar articles
-
Anti-cancer effects of the aqueous extract of Orostachys japonica A. Berger on 5-fluorouracil-resistant colorectal cancer via MAPK signalling pathways in vitro and in vivo.J Ethnopharmacol. 2021 Nov 15;280:114412. doi: 10.1016/j.jep.2021.114412. Epub 2021 Jul 12. J Ethnopharmacol. 2021. PMID: 34265383
-
Syndecan-2, negatively regulated by miR-20b-5p, contributes to 5-fluorouracil resistance of colorectal cancer cells via the JNK/ERK signaling pathway.Acta Biochim Biophys Sin (Shanghai). 2021 Nov 10;53(11):1547-1557. doi: 10.1093/abbs/gmab124. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34596215
-
TIMP-2 regulates 5-Fu resistance via the ERK/MAPK signaling pathway in colorectal cancer.Aging (Albany NY). 2022 Jan 12;14(1):297-315. doi: 10.18632/aging.203793. Epub 2022 Jan 12. Aging (Albany NY). 2022. PMID: 35022331 Free PMC article.
-
Gypenosides Synergistically Enhances the Anti-Tumor Effect of 5-Fluorouracil on Colorectal Cancer In Vitro and In Vivo: A Role for Oxidative Stress-Mediated DNA Damage and p53 Activation.PLoS One. 2015 Sep 14;10(9):e0137888. doi: 10.1371/journal.pone.0137888. eCollection 2015. PLoS One. 2015. PMID: 26368019 Free PMC article.
-
Microbial metabolite sodium butyrate enhances the anti-tumor efficacy of 5-fluorouracil against colorectal cancer by modulating PINK1/Parkin signaling and intestinal flora.Sci Rep. 2024 Jun 6;14(1):13063. doi: 10.1038/s41598-024-63993-x. Sci Rep. 2024. PMID: 38844824 Free PMC article.
Cited by
-
Synergistic ROS/enzyme dual-responsive oral drug delivery system: A novel multi-mechanistic platform for spatiotemporal control and overcoming drug resistance in colorectal cancer therapy.Mater Today Bio. 2025 May 30;33:101920. doi: 10.1016/j.mtbio.2025.101920. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40528838 Free PMC article.
References
-
- The Korean Statistical Information Service Current status of cancer incidence and cancer-related death. 2021. https://www.index.go.kr/unity/portal/main/EachDtlPageDetail.do?idxcd=2770. [Accessed 2023].
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
