Chloride channel-3 regulates sodium-iodide symporter expression and localization in the thyroids of mice on a high-iodide diet
- PMID: 40191796
- PMCID: PMC11968397
- DOI: 10.3389/fnut.2025.1537221
Chloride channel-3 regulates sodium-iodide symporter expression and localization in the thyroids of mice on a high-iodide diet
Abstract
Introduction: Certain chloride channels and H+/Cl- antiporters, such as chloride channel 3 (ClC-3), are expressed at the apical pole of thyrocytes, facilitating iodide (I-) efflux. However, the relationship between ClC-3 and I- uptake remains unclear. Additionally, whether ClC-3 and reactive oxygen species (ROS) regulate sodium-iodide symporter (NIS) expression and localization under excessive I- conditions remain underexplored.
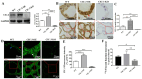
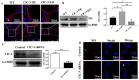
Methods: The expression and localization of ClC-3 in wild-type (WT), ClC-3 overexpression (OE) and ClC-3 knockout (KO) were detected by Western blotting (WB), immunohistochemistry and immunofluorescence, respectively. The 131I uptake of the thyroid was measured by thyroid function instrument. The expression and localization of NIS in normal and high iodide diet were detected, respectively. The role of ROS in the regulation of NIS by ClC-3 was observed.
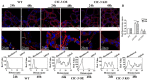
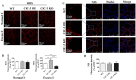
Results: ClC-3 expressions in thyrocytes were primarily localized to the basolateral and lateral membranes, in both ClC-3 OE and WT mice groups under normal I- conditions. I- uptake was significantly higher in WT and ClC-3 OE mice than in the ClC-3 KO mice under normal I- conditions. The ClC-3 OE group exhibited a higher number of thyroid follicles with elevated NIS expression in the basolateral and lateral membranes than the WT and KO groups. In the ClC-3 KO group, the NIS was predominantly localized in the cytoplasm. In the WT group, NIS fluorescence intensity at the basolateral and lateral membranes increased after 48 h of excessive iodide exposure compared to 24 h. In ClC-3 OE mice, NIS, initially localized intracellularly after 24 h of excessive iodide exposure, was almost fully reintegrated into the basolateral and lateral membranes after 48 h. In contrast, in ClC-3 KO mice, NIS remained primarily cytoplasmic, with no significant change between 24 h and 48 h of I- excess. ROS fluorescence intensity was significantly higher in the ClC-3 OE group than those in the WT and KO groups after 24 h of I- excess. Pre-inhibition of ROS showed no significant differences in NIS localization or expression among the three groups after 24 h of I- excess.
Discussion: These findings suggest that ClC-3 may regulate NIS function via ROS signaling under excessive iodide conditions.
Keywords: basolateral and lateral membranes; chloride channel 3; iodide conditions; iodide excess; reactive oxygen species; sodium-iodide symporter; thyroid gland.
Copyright © 2025 Yu, Deng, Wang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

