miR-627-5p inhibits malignant progression of cervical cancer by targeting ANGPTL4
- PMID: 40191921
- PMCID: PMC12038335
- DOI: 10.4081/ejh.2025.4161
miR-627-5p inhibits malignant progression of cervical cancer by targeting ANGPTL4
Abstract
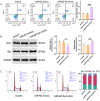
In recent years, accumulating evidence has highlighted the critical role of miR-627-5p in the occurrence and progression of various cancers. However, its specific role and mechanism in cervical cancer (CC) remain unclear. This study aimed to elucidate the mechanism by which miR-627-5p inhibits the malignant progression of CC and assess its potential clinical implications. In C33A cells, the mRNA expression levels of ANGPTL4 and miR-627-5p were analyzed using qRT-PCR. The miR-627-5p mimics and their control (miR-NC) were transfected into C33A cells to determine whether miR-627-5p directly regulates ANGPTL4 expression. A comprehensive suite of assays, including CCK-8, migration, transwell, flow cytometry, and Western blotting, was conducted to evaluate how miR-627-5p modulates the malignant biological behavior of CC cells. Rescue experiments were performed by overexpressing ANGPTL4. In C33A cells, miR-627-5p expression was reduced, whereas ANGPTL4 expression was elevated. Further analysis confirmed that miR-627-5p negatively regulates ANGPTL4 by directly targeting its 3'-UTR. Functional assays demonstrated that miR-627-5p inhibits proliferation, invasion, migration, and epithelial-mesenchymal transition (EMT) while promoting apoptosis and S-phase arrest in C33A cells, effects that were reversed by ANGPTL4 overexpression. These findings highlight the potential of miR-627-5p as both a biomarker and a therapeutic target for CC. By inhibiting EMT and regulating ANGPTL4 expression, miR-627-5p may provide a novel avenue for improving therapeutic strategies, particularly in advanced or metastatic CC. Moreover, miRNA-based therapies, supported by advanced delivery systems such as nanoparticle carriers, could enhance the stability and precision of miR-627-5p applications. This study lays the groundwork for future research integrating miR-627-5p into precision medicine approaches for CC treatment.
Conflict of interest statement
The authors declare no conflict of interest regarding the present study.
Figures




Similar articles
-
Effects of miR-202-5p silencing PIK3CA gene expression on proliferation, invasion, and epithelial-mesenchymal transition of cervical cancer SiHa cells through inhibiting PI3K/Akt/mTOR signaling pathway activation.Mol Cell Biochem. 2021 Nov;476(11):4031-4044. doi: 10.1007/s11010-021-04211-4. Epub 2021 Jul 9. Mol Cell Biochem. 2021. PMID: 34244973
-
LncRNA TDRG1 promotes the proliferation, migration, and invasion of cervical cancer cells by sponging miR-214-5p to target SOX4.J Recept Signal Transduct Res. 2020 Jun;40(3):281-293. doi: 10.1080/10799893.2020.1731537. Epub 2020 Feb 27. J Recept Signal Transduct Res. 2020. PMID: 32106739
-
CircVIRMA enhances cell malignant behavior by governing the miR-452-5p/CREBRF pathway in cervical cancer.Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov;397(11):8825-8838. doi: 10.1007/s00210-024-03159-8. Epub 2024 Jun 8. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38850300
-
Effects of miR-9-5p on the migration, invasion and epithelial-mesenchymal transition process in cervical squamous cell carcinoma.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 Jan 28;48(1):15-23. doi: 10.11817/j.issn.1672-7347.2023.210773. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 36935173 Free PMC article. Chinese, English.
-
ATG9A modulated by miR-195-5p can boost the malignant progression of cervical cancer cells.Epigenetics. 2023 Dec;18(1):2257538. doi: 10.1080/15592294.2023.2257538. Epub 2023 Oct 2. Epigenetics. 2023. PMID: 37782756 Free PMC article.
References
-
- Lin S, Zhu J, Mao X, Lin G, Yang D, Guan Y, et al. . GRAP2: a novel immune-related prognosis biomarker in cervical cancer. Clin Exp Obstet Gynecol 2023;50: 7.
-
- D'Augè TG, Giannini A, Bogani G, Di Dio C, Lagan AS, Di Donato V, et al. . Prevention, screening, treatment and follow-up of gynecological cancers. State of art and future perspectives. Clin Exp Obstet Gynecol 2023;50:160.
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. - PubMed
-
- Adiga D, Eswaran S, Pandey D, Sharan K, Kabekkodu SP. Molecular landscape of recurrent cervical cancer. Crit Rev Oncol Hematol 2021;157:103178. - PubMed

