Multi-response phylogenetic mixed models: concepts and application
- PMID: 40192008
- PMCID: PMC12120399
- DOI: 10.1111/brv.70001
Multi-response phylogenetic mixed models: concepts and application
Abstract
The scale and resolution of trait databases and molecular phylogenies is increasing rapidly. These resources permit many open questions in comparative biology to be addressed with the right statistical tools. Multi-response (MR) phylogenetic mixed models (PMMs) offer great potential for multivariate analyses of trait evolution. While flexible and powerful, these methods are not often employed by researchers in ecology and evolution, reflecting a specialised and technical literature that creates barriers to usage for many biologists. Here we present a practical and accessible guide to MR-PMMs. We begin with a review of single-response (SR) PMMs to introduce key concepts and outline the limitations of this approach for characterising patterns of trait coevolution. We emphasise MR-PMMs as a preferable approach for analyses involving multiple species traits, due to the explicit decomposition of trait covariances. We discuss multilevel models, multivariate models of evolution, and extensions to non-Gaussian response traits. We highlight techniques for causal inference using graphical models, as well as advanced topics including prior specification and latent factor models. Using simulated data and visual examples, we discuss interpretation, prediction, and model validation. We implement many of the techniques discussed in example analyses of plant functional traits to demonstrate the general utility of MR-PMMs in handling complex real-world data sets. Finally, we discuss the emerging synthesis of comparative techniques made possible by MR-PMMs, highlight strengths and weaknesses, and offer practical recommendations to analysts. To complement this material, we provide online tutorials including side-by-side model implementations in two popular R packages, MCMCglmm and brms.
Keywords: evolutionary ecology; generalised linear mixed models; multivariate statistics; phylogenetic comparative methods; trait evolution; variance partitioning.
© 2025 The Author(s). Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures
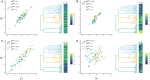
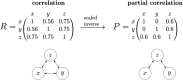
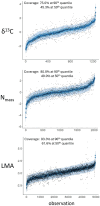
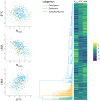
Similar articles
-
Of Traits and Trees: Probabilistic Distances under Continuous Trait Models for Dissecting the Interplay among Phylogeny, Model, and Data.Syst Biol. 2021 Jun 16;70(4):660-680. doi: 10.1093/sysbio/syab009. Syst Biol. 2021. PMID: 33587145 Free PMC article.
-
Estimating the Effect of Competition on Trait Evolution Using Maximum Likelihood Inference.Syst Biol. 2016 Jul;65(4):700-10. doi: 10.1093/sysbio/syw020. Epub 2016 Mar 9. Syst Biol. 2016. PMID: 26966005
-
No substitute for real data: A cautionary note on the use of phylogenies from birth-death polytomy resolvers for downstream comparative analyses.Evolution. 2015 Dec;69(12):3207-16. doi: 10.1111/evo.12817. Evolution. 2015. PMID: 26552857
-
Translational Metabolomics of Head Injury: Exploring Dysfunctional Cerebral Metabolism with Ex Vivo NMR Spectroscopy-Based Metabolite Quantification.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. PMID: 26269925 Free Books & Documents. Review.
-
Multivariate Phylogenetic Comparative Methods: Evaluations, Comparisons, and Recommendations.Syst Biol. 2018 Jan 1;67(1):14-31. doi: 10.1093/sysbio/syx055. Syst Biol. 2018. PMID: 28633306 Review.
Cited by
-
Non-Native Woody Plant Species Show Different Leaf Functional Traits and Herbivory Levels From Native Ones in the Urban Areas of Beijing, China.Ecol Evol. 2025 Aug 8;15(8):e71947. doi: 10.1002/ece3.71947. eCollection 2025 Aug. Ecol Evol. 2025. PMID: 40785986 Free PMC article.
References
-
- Adams, D. C. (2014). A method for assessing phylogenetic least squares models for shape and other high‐dimensional multivariate data. Evolution 68, 2675–2688. - PubMed
-
- Adams, D. C. & Collyer, M. L. (2018). Multivariate phylogenetic comparative methods: evaluations, comparisons, and recommendations. Systematic Biology 67, 14–31. - PubMed
-
- Adams, D. C. & Collyer, M. L. (2019). Phylogenetic comparative methods and the evolution of multivariate phenotypes. Annual Review of Ecology, Evolution, and Systematics 50, 405–425.
-
- Akaike, H. (1973). Tsahkadsor, 1971, pp. 267–281. Academiai Kiado, Budapest.
-
- Arlot, S. & Celisse, A. (2010). A survey of cross‐validation procedures for model selection. Statistics Surveys 4, 40–79.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

