Aerobic Exercise Restores Hippocampal Neurogenesis and Cognitive Function by Decreasing Microglia Inflammasome Formation Through Irisin/NLRP3 Pathway
- PMID: 40192010
- PMCID: PMC12266781
- DOI: 10.1111/acel.70061
Aerobic Exercise Restores Hippocampal Neurogenesis and Cognitive Function by Decreasing Microglia Inflammasome Formation Through Irisin/NLRP3 Pathway
Abstract
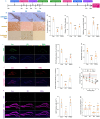
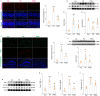
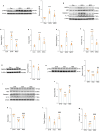
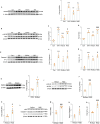
Persistent microglial inflammation is a detrimental contributor to the progression of Parkinson disease (PD) pathology and related issues such as impaired adult hippocampal neurogenesis (AHN) and cognition. We conducted a 10-week exercise program with MPTP-treated mice to determine whether neuroinflammation can be addressed by aerobic exercise and elucidate its underlying regulatory mechanisms. Ten weeks of exercise significantly reduced PD-related pathology and enhanced AHN and memory. These changes were linked to a reduction in neuronal apoptosis, microglial inflammation, and NLRP3 inflammasome activation. In cultured microglia, fibril α-synuclein reduced FNDC5/irisin protein levels and induced NLRP3 inflammasome formation and IL-1β production, which could be diminished by recombinant irisin treatment. Interestingly, "runner serum" isolated from exercising rodents enhanced FNDC5/irisin expression and reduced NLRP3 inflammasome components and IL-1β secretion in α-synuclein-treated microglia. These effects could be diminished by blocking irisin signaling with cyclo RGDyk or NLRP3 agonist, nigericin sodium salt. Exercise-induced neuroprotective effects were weakened by treatment of MPTP-treated mice with cyclo RGDyk. In contrast, systematic administration of irisin partially replicated the beneficial effects of exercise on PD pathology, AHN, and memory function. As a nonpharmacological strategy, aerobic exercise effectively addresses PD pathology and preserves adult neurogenesis and cognition by mitigating microglial inflammation via mediating irisin/NLRP3 inflammasome pathways.
Keywords: FNDC5/irisin; NLRP3 inflammasome; Parkinson disease; aerobic exercise; cognition; hippocampal neurogenesis.
© 2025 The Author(s). Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Irisin ameliorates cognitive impairment in a lipopolysaccharide-induced neuroinflammation mouse model by inhibiting the NLRP3 inflammasome pathway in microglia.Neuropharmacology. 2025 Nov 1;278:110572. doi: 10.1016/j.neuropharm.2025.110572. Epub 2025 Jun 23. Neuropharmacology. 2025. PMID: 40562230
-
Exercise Ameliorates Dysregulated Mitochondrial Fission, Mitochondrial Respiration, and Neuronal Apoptosis in Parkinson's Disease Mice via the Irisin/AMPK/SIRT1 Pathway.Mol Neurobiol. 2025 Jul;62(7):8843-8856. doi: 10.1007/s12035-025-04801-z. Epub 2025 Mar 6. Mol Neurobiol. 2025. PMID: 40048058
-
Exercise-induced irisin ameliorates cognitive impairment following chronic cerebral hypoperfusion by suppressing neuroinflammation and hippocampal neuronal apoptosis.J Neuroinflammation. 2025 Jun 28;22(1):168. doi: 10.1186/s12974-025-03493-5. J Neuroinflammation. 2025. PMID: 40581647 Free PMC article.
-
New Insights on the Potential Role of Pyroptosis in Parkinson's Neuropathology and Therapeutic Targeting of NLRP3 Inflammasome with Recent Advances in Nanoparticle-Based miRNA Therapeutics.Mol Neurobiol. 2025 Jul;62(7):9365-9384. doi: 10.1007/s12035-025-04818-4. Epub 2025 Mar 18. Mol Neurobiol. 2025. PMID: 40100493 Review.
-
Mechanisms underlying the interplay between autophagy and the inflammasome in age-related diseases: Implications for exercise immunology.Ageing Res Rev. 2025 Aug;110:102821. doi: 10.1016/j.arr.2025.102821. Epub 2025 Jul 1. Ageing Res Rev. 2025. PMID: 40609647 Review.
Cited by
-
New perspectives on molecular mechanisms underlying exercise-induced benefits in Parkinson's disease.NPJ Parkinsons Dis. 2025 Aug 23;11(1):256. doi: 10.1038/s41531-025-01113-w. NPJ Parkinsons Dis. 2025. PMID: 40849310 Review.
References
-
- Aaron, K. , and Brendon S.. 2021. “Exercise and Inflammation.” In Immuno‐Psychiatry: Facts and Prospects, edited by Berk M., Leboyer M., and Sommer I. E., 431–444. Springer International Publishing.
-
- Ahmadi, A. , Kashef M., Rajabi H., and Salehpour M.. 2022. “Effects of Exercise Preconditioning on NLRP3 and Mitochondrial Fission in Isoproterenol‐Induced Myocardial Infarcted Rats.” Comparative Clinical Pathology 32: 289–297. 10.1007/s00580-022-03397-3. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

