Application and future prospects of bispecific antibodies in the treatment of non-small cell lung cancer
- PMID: 40192238
- PMCID: PMC12032835
- DOI: 10.20892/j.issn.2095-3941.2024.0470
Application and future prospects of bispecific antibodies in the treatment of non-small cell lung cancer
Abstract
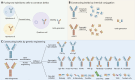
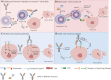
As the leading cause of cancer-related deaths, lung cancer remains a noteworthy threat to human health. Although immunotherapies, such as immune checkpoint inhibitors (ICIs), have significantly increased the efficacy of lung cancer treatment, a significant percentage of patients are not sensitive to immunotherapies and patients who initially respond to treatment can quickly develop acquired drug resistance. Bispecific antibodies (bsAbs) bind two different antigens or epitopes simultaneously and have been shown to enhance antitumor efficacy with suitable safety profiles, thus attracting increasing attention as novel antitumor therapies. At present, in addition to the approved bsAb, amivantamab, three novel bsAbs (KN046, AK112, and SHR-1701) are being evaluated in phase 3 clinical trials and many bsAbs are being evaluated in phase 1/2 clinical trials for patients with non-small cell lung cancer (NSCLC). Herein we present the structure, classification, and mechanism of action underlying bsAbs in NSCLC and introduce related clinical trials. Finally, we discuss challenges, potential solutions, and future prospects in the context of cancer treatment with bsAbs.
Keywords: Bispecific antibody; challenges; non-small cell lung cancer; novel antitumor therapy; structure.
Copyright © 2025 The Authors.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Figures


Similar articles
-
Unveiling the Synergistic Potential: Bispecific Antibodies in Conjunction with Chemotherapy for Advanced Non-Small-Cell Lung Cancer Treatment.Curr Oncol. 2025 Mar 31;32(4):206. doi: 10.3390/curroncol32040206. Curr Oncol. 2025. PMID: 40277763 Free PMC article. Review.
-
Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET.J Biol Chem. 2021 Jan-Jun;296:100641. doi: 10.1016/j.jbc.2021.100641. Epub 2021 Apr 8. J Biol Chem. 2021. PMID: 33839159 Free PMC article.
-
A novel bispecific c-MET/CTLA-4 antibody targetting lung cancer stem cell-like cells with therapeutic potential in human non-small-cell lung cancer.Biosci Rep. 2019 May 31;39(5):BSR20171278. doi: 10.1042/BSR20171278. Print 2019 May 31. Biosci Rep. 2019. PMID: 29187584 Free PMC article.
-
Bispecific monoclonal antibodies for targeted immunotherapy of solid tumors: Recent advances and clinical trials.Int J Biol Macromol. 2021 Jan 15;167:1030-1047. doi: 10.1016/j.ijbiomac.2020.11.058. Epub 2020 Nov 14. Int J Biol Macromol. 2021. PMID: 33197478 Review.
-
Amivantamab: First Approval.Drugs. 2021 Jul;81(11):1349-1353. doi: 10.1007/s40265-021-01561-7. Drugs. 2021. PMID: 34292533 Review.
Cited by
-
Rapid and specific immunoPET imaging of Nectin-4 in gastric cancer and non-small cell lung cancer using [64Cu]Cu-NOTA-EV-F(ab')2.Eur J Nucl Med Mol Imaging. 2025 Jun 21:10.1007/s00259-025-07402-z. doi: 10.1007/s00259-025-07402-z. Online ahead of print. Eur J Nucl Med Mol Imaging. 2025. PMID: 40542857
-
Emerging IO checkpoints in gastrointestinal oncology.Front Immunol. 2025 Jul 24;16:1575713. doi: 10.3389/fimmu.2025.1575713. eCollection 2025. Front Immunol. 2025. PMID: 40777027 Free PMC article. Review.
References
-
- Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27:1345–56. - PubMed
-
- Syed YY. Amivantamab: first approval. Drugs. 2021;81:1349–53. - PubMed
-
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
