Safety, pharmacokinetics, and biological activity of CD4-mimetic BNM-III-170 in SHIV-infected rhesus macaques
- PMID: 40192306
- PMCID: PMC12090809
- DOI: 10.1128/jvi.00062-25
Safety, pharmacokinetics, and biological activity of CD4-mimetic BNM-III-170 in SHIV-infected rhesus macaques
Abstract
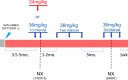
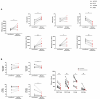
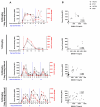
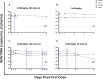
Anti-HIV-1 antibodies capable of mediating ADCC are elicited by the majority of people with HIV-1 and preferentially target the "open," CD4-bound conformation of HIV-1 envelope glycoproteins (Env). However, due to the "closed" conformation sampled by unliganded HIV-1-Envs, these antibodies are ineffective at eliminating infected cells. BNM-III-170 is a small-molecule CD4-mimetic compound that binds the Phe43 cavity of the gp120 subunit of Env, forcing Env to "open up," thus exposing epitopes targeted by CD4-induced (CD4i), ADCC-mediating antibodies. Here, we assessed the safety, pharmacokinetics, and biological activity of BNM-III-170 in uninfected and SHIV-AD8-EO-infected rhesus macaques (RMs). In uninfected RMs, single subcutaneous administrations of 3-36 mg/kg BNM-III-170 were well-tolerated, with serum half-lives ranging from 3 to 6 h. In SHIV-infected RMs, four different regimens were evaluated: 2 × 36 mg/kg daily, 1 × 24 mg/kg, 3 × 36 mg/kg every 7 days, and 3 × 36 mg/kg every 3 days. While toxicity was observed with daily doses, all other regimens demonstrated reasonable safety profiles. No changes in plasma viral loads were observed in SHIV-infected RMs following any of the evaluated BNM-III-170 dosing regimens. However, plasma collected following BNM-III-170 administration was shown to have increased binding to infected cells and to sensitize SHIV AD8-EO virions to neutralization by otherwise non-neutralizing antibodies. In addition, the plasma of treated animals mediated ADCC in the presence of BNM-III-170. These results establish a well-tolerated BNM-III-170 dosing regimen in SHIV-infected RMs and serve as proof of concept for its biological activity in promoting the targeting of infected cells by CD4i ADCC-mediating antibodies. Thus, they inform future studies evaluating CD4mc treatment in ART-treated animals.IMPORTANCEA therapeutic regimen able to eradicate or functionally cure HIV-1 remains elusive and may require a "shock-and-kill" approach to reactivate and then purge the latent HIV-1 reservoir. The small-molecule CD4-mimetic compound BNM-III-170 has previously been shown to (i) sensitize HIV-1-infected cells to ADCC mediated by plasma from people with HIV-1 (PWH) in vitro and (ii) significantly delay the time to viral rebound following ART interruption when combined with anti-CoRBS + anti-cluster A Abs or plasma from PWH in humanized mice. To evaluate the use of BNM-III-170 as part of a kill approach, we characterized the safety, pharmacokinetics, and biological activity of BNM-III-170 in uninfected and SHIV-infected RMs. Our study identifies a tolerable BNM-III-170 dosing regimen in SHIV-infected RMs and provides insights into its antiviral activities; as such, it informs future studies evaluating the efficacy of BNM-III-170 in reducing the viral reservoir.
Keywords: ADCC; CD4 mimetic; human immunodeficiency virus; in vivo therapeutic strategies; nonhuman primate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The combination of three CD4-induced antibodies targeting highly conserved Env regions with a small CD4-mimetic achieves potent ADCC activity.J Virol. 2024 Oct 22;98(10):e0101624. doi: 10.1128/jvi.01016-24. Epub 2024 Sep 9. J Virol. 2024. PMID: 39248460 Free PMC article.
-
CD4-mimetics sensitize HIV-infected cells to ADCC mediated by plasma from persons with early-stage HIV-1 infection.J Virol. 2025 Jul 21:e0085825. doi: 10.1128/jvi.00858-25. Online ahead of print. J Virol. 2025. PMID: 40689664
-
Assessing the impact of autologous virus neutralizing antibodies on viral rebound time in postnatally SHIV-infected ART-treated infant rhesus macaques.Epidemics. 2024 Sep;48:100780. doi: 10.1016/j.epidem.2024.100780. Epub 2024 Jun 27. Epidemics. 2024. PMID: 38964130 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Structured treatment interruptions (STI) in chronic unsuppressed HIV infection in adults.Cochrane Database Syst Rev. 2006 Jul 19;2006(3):CD006148. doi: 10.1002/14651858.CD006148. Cochrane Database Syst Rev. 2006. PMID: 16856117 Free PMC article.
References
-
- UNAIDS . 2024. 2024 Global AIDS Report — The Urgency of Now: AIDS at a Crossroads 2024. Available from: https://www.unaids.org/sites/default/files/media_asset/2024-unaids-globa...
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5:512–517. doi:10.1038/8394 - DOI - PubMed
-
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183–188. doi:10.1038/387183a0 - DOI - PubMed
-
- Perreau M, Savoye A-L, De Crignis E, Corpataux J-M, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. 2013. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 210:143–156. doi:10.1084/jem.20121932 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

