Nicotinamide Riboside Mitigates Retinal Degeneration by Suppressing Damaged DNA-Stimulated Microglial Activation and STING-Mediated Pyroptosis
- PMID: 40192637
- PMCID: PMC11980955
- DOI: 10.1167/iovs.66.4.14
Nicotinamide Riboside Mitigates Retinal Degeneration by Suppressing Damaged DNA-Stimulated Microglial Activation and STING-Mediated Pyroptosis
Abstract
Purpose: Microglial activation plays a pivotal role in the pathogenesis of retinal degeneration, contributing to neuroinflammation within the retina. Previous studies identified that nicotinamide riboside (NR) mitigated light-induced retinal degeneration (LIRD) and inhibited microglial activation. The cGAS-STING signaling pathway has been recognized as a key mediator of inflammation in response to cellular stress and tissue damage. This study further explores the regulatory impact of NR on microglial activation and STING-mediated pyroptosis in retinal degeneration.
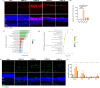
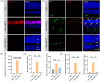
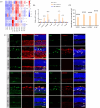
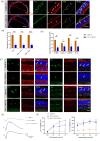
Methods: Balb/c mice were subjected to bright light exposure to induce retinal degeneration. Bioinformatics analysis was used to identify the upregulated key genes and signaling pathways involved in the progression of retinal degeneration, based on mouse transcriptomes from the LIRD model. Molecular biology techniques and immunofluorescence staining were used to assess cGAS-STING activation and expression of pyroptosis-associated molecules. Retinal function, photoreceptor apoptosis and inflammatory response were evaluated in the presence and absence of NR supplementation.
Results: Exposure to bright light resulted in mitochondrial dysfunction and the release of dsDNA, significantly triggering the activation of cGAS-STING pathway and microglial pyroptosis. In contrast, NR treatment preserved mitochondrial biosynthesis, inhibited STING expression in reactive microglia, and dampened the pro-inflammatory response. Additionally, intraperitoneal administration of the STING inhibitor H151 reduced light-induced microglial activation and pyroptosis, while improving retinal function and promoting photoreceptor survival.
Conclusions: These findings suggest that NR confers neuroprotection by attenuating damaged DNA-triggered STING-mediated microglial activation and pyroptosis. Targeting the cGAS-STING pathway presents a promising therapeutic avenue for retinal degeneration.
Conflict of interest statement
Disclosure:
Figures


References
-
- Fletcher EL. Mechanisms of photoreceptor death during retinal degeneration. Optom Vis Sci. 2010; 87: 269–275. - PubMed
-
- Gagliardi G, Ben M'Barek K, Goureau O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: a pluripotent stem cell-based approach. Prog Retin Eye Res. 2019; 71: 1–25. - PubMed
-
- Yoshida N, Ikeda Y, Notomi S, et al. .. Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013; 120: 100–105. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

