Micro-proteomics reveals distinct protein profiles and SPARC/FGF2/CDH1 regulation of human Sertoli cells between Sertoli cell-only syndrome and normal men
- PMID: 40192810
- PMCID: PMC11977051
- DOI: 10.1007/s00018-025-05678-w
Micro-proteomics reveals distinct protein profiles and SPARC/FGF2/CDH1 regulation of human Sertoli cells between Sertoli cell-only syndrome and normal men
Abstract
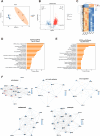
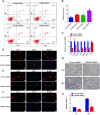
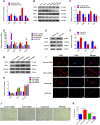
Sertoli cell-only syndrome (SCOS) is one of the most severe non-obstructive azoospermia (NOA) types, since only Sertoli cells with not any male germ cells exist with the seminiferous tubules. As such, it is of particular significance to elucidate molecular mechanisms underlying SCOS for improving the diagnosis and treatment strategies for this disease. Due to the difficulties in obtaining sufficient human testicular tissues and the limited availability of human cells, the traditional proteomics is inadequate for comparing the differences in large scale of protein expression patterns of human Sertoli cells between SCOS and normal men. To solve this issue on the requirement of large amount of cell numbers, we employed micro-proteomics to reveal distinct global protein expression profiles of human Sertoli cells between SCOS and obstructive azoospermia (OA) with normal spermatogenesis utilizing single human Sertoli cells. We found a significant downregulation of proteins involved in cell adhesion pathways in SCOS Sertoli cells, whereas proteins related to apoptosis were markedly upregulated. Interestingly, we identified the lower expression of SPARC (secreted protein acidic and rich in cysteine) and the higher expression of FGF2 (fibroblast growth factor 2) in human Sertoli cells of the SCOS compared to normal men. SPARC silencing led to upregulation of FGF2 in human Sertoli cells, and SPARC may be associated with the occurrence of SCOS and serves as a reliable marker for the diagnosis of this disease. This study thus comprehensively offers the proteomic landscape of human Sertoli cells in the testes of SCOS patients and it sheds a novel insight into the pathogenesis of SCOS.
Keywords: CDH1; FGF2; Human Sertoli cells; Micro-proteomics; SPARC; Sertoli cell-only syndrome.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Review Committee of Hunan Normal University (ethics statement: no: 2021227), and the informed consent was obtained for research purposes only. Consent for publication: All authors approved this manuscript for publication. Competing interests: The authors declared no competing financial interest.
Figures




Similar articles
-
HnRNPL as a key factor in spermatogenesis: Lesson from functional proteomic studies of azoospermia patients with sertoli cell only syndrome.J Proteomics. 2012 Jun 6;75(10):2879-91. doi: 10.1016/j.jprot.2011.12.040. Epub 2012 Jan 10. J Proteomics. 2012. PMID: 22245417
-
Fibroblast growth factor-5 promotes spermatogonial stem cell proliferation via ERK and AKT activation.Stem Cell Res Ther. 2019 Jan 22;10(1):40. doi: 10.1186/s13287-019-1139-7. Stem Cell Res Ther. 2019. PMID: 30670081 Free PMC article.
-
Up-regulation of SOX9 in sertoli cells from testiculopathic patients accounts for increasing anti-mullerian hormone expression via impaired androgen receptor signaling.PLoS One. 2013 Oct 1;8(10):e76303. doi: 10.1371/journal.pone.0076303. eCollection 2013. PLoS One. 2013. PMID: 24098470 Free PMC article.
-
Sertoli cell-only syndrome: advances, challenges, and perspectives in genetics and mechanisms.Cell Mol Life Sci. 2023 Feb 23;80(3):67. doi: 10.1007/s00018-023-04723-w. Cell Mol Life Sci. 2023. PMID: 36814036 Free PMC article. Review.
-
Sertoli cell-only syndrome: etiology and clinical management.J Assist Reprod Genet. 2021 Mar;38(3):559-572. doi: 10.1007/s10815-021-02063-x. Epub 2021 Jan 11. J Assist Reprod Genet. 2021. PMID: 33428073 Free PMC article. Review.
References
-
- Hovatta O (1996) Sertoli cell only syndrome. Hum Reprod 11:229. 10.1093/oxfordjournals.humrep.a019025 - PubMed
-
- Silber SJ (1996) Sertoli cell only syndrome. Hum Reprod 11:229. 10.1093/oxfordjournals.humrep.a019026 - PubMed
-
- Ezeh UI, Moore HD, Cooke ID (1998) A prospective study of multiple needle biopsies versus a single open biopsy for testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod 13:3075–3080. 10.1093/humrep/13.11.3075 - PubMed
-
- Silber SJ (2000) Microsurgical TESE and the distribution of spermatogenesis in non-obstructive azoospermia. Hum Reprod 15:2278–2284. 10.1093/humrep/15.11.2278 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

