An Open-label, Randomized Study of Melphalan/Hepatic Delivery System Versus Best Alternative Care in Patients with Unresectable Metastatic Uveal Melanoma
- PMID: 40192993
- PMCID: PMC12130151
- DOI: 10.1245/s10434-025-17231-x
An Open-label, Randomized Study of Melphalan/Hepatic Delivery System Versus Best Alternative Care in Patients with Unresectable Metastatic Uveal Melanoma
Abstract
Background: Metastatic uveal melanoma (mUM) has a poor prognosis, with liver metastases typically presenting a therapeutic challenge. Melphalan/Hepatic Delivery System (Melphalan/HDS) is a drug/medical device combination used for liver-directed treatment of unresectable mUM patients. This study assessed efficacy and safety of Melphalan/HDS versus best alternative care (BAC).
Methods: Eligible patients with unresectable mUM were randomized (1:1) to receive Melphalan/HDS (3 mg/kg ideal body weight) once every 6 to 8 weeks for a maximum of 6 cycles or BAC. Due to slow enrollment and patient reluctance to receive BAC treatment, the study design was amended to a single-arm Melphalan/HDS study, and all efficacy analyses of the randomized study were treated as exploratory.
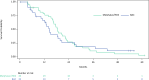
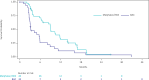
Results: The study enrolled 85 patients. Eligible patients were randomized to receive Melphalan/HDS (n = 43) or BAC (n = 42), and 72 patients received study treatment (Melphalan/HDS [n = 40]; BAC [n = 32]). Exploratory analyses of efficacy endpoints showed numerical differences consistently favoring the Melphalan/HDS arm versus BAC (median overall survival: 18.5 vs. 14.5 months; median progression-free survival: 9.1 vs. 3.3 months; objective response rate: 27.5% vs. 9.4%; and disease control rate: 80.0% vs. 46.9%). Serious adverse events (SAEs) occurred in 51.2% of Melphalan/HDS and in 21.9% of BAC patients. The most common (>5%) SAEs included thrombocytopenia (19.5%), neutropenia (9.8%), leukopenia (9.8%) and febrile neutropenia (7.3%) in Melphalan/HDS patients and cholecystitis, nausea and vomiting (6.3% each) in BAC patients. No treatment-related deaths were observed.
Conclusion: Treatment with Melphalan/HDS shows clinically meaningful efficacy and demonstrates a favorable benefit-risk profile in patients with unresectable mUM as compared to BAC.
Keywords: Liver-directed therapy; Melphalan/Hepatic Delivery System; Metastatic uveal melanoma; Ocular melanoma; Percutaneous hepatic perfusion.
© 2025. The Author(s).
Conflict of interest statement
Disclosure: JZ–global principal investigator in the FOCUS phase 3 study and serves on Delcath Systems Medical Advisory Board. Education and Training grant through Delcath. Has consulting agreements and/or participated on advisory board panels with Merit Medical, Replumune, Merck, Philogen, Castle Biosciences and Menarini Silicon Biosystems. MO–Advisory Board: Delcath, Replimune. Consultant: Immunocore. Steering Committee: Ideaya. Speaker: Immunocore. DE–Consulting fees from Delcath. EG–Institutional funding for conducting the Phase 3 FOCUS study. JH–Consultant: Delcath. ER–Medical University of Graz received payments for conducting the Phase 3 FOCUS study. SO–Advice: Immunocore, Delcath, Janssen. Speakers’ bureau: Immunocore. Honoraria for presentations: MSD, AstraZeneca, Merck; Travel funding: Merck, Pfizer, Ipsen. GB–Clinical trial funding paid to institution from Replimune, Checkmate pharmaceuticals, Philogen, Delcath. Advisory board: BMS. AG–University Hospital Würzburg received institutional payments for conducting the Phase 3 FOCUS study. RD–Consulting and/or advisory relationships with Novartis, Merck Sharp & Dhome, Bristol-Myers Squibb, Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator, T3 Pharma, MaxiVAX SA, Pfizer, Simcere and touchIME. AA–Consulting or advisory role: Pierre Fabre, Novartis, Roche, BMS, MSD, Biontech. Speakers’ Bureau: Pierre Fabre, Novartis, Roche, BMS, MSD. Travel, Accommodations, Expenses: BMS, MSD. SF–Liverpool University Hospitals NHS Trust received institutional support for conducting this Phase 3 FOCUS study from the trial sponsor. JS–Advisory boards: Delcath, Replimune and Immunocore. Grant funding: Astra Zeneca and BMS (paid to institution). Travel and conference attendance: MSD, BMS and Replimune. SH - Speakers Honoraria: BMS, MSD, Pierre Fabre, Sanofi, Immunocore. MW–Institutional (University Hospital Southampton) funding for conducting a commercial trial. Personal funding for participation in advisory board for Delcath. JJ–Employee of Delcath.
Figures

References
-
- Carvajal RD, Sacco JJ, Jager MJ, Eschelman DJ, Bagge RO, Harbour JW, et al. Advances in the clinical management of uveal melanoma. Nat Rev Clin Oncol. 2023;20(2):99–115. 10.1038/s41571-022-00714-1. - PubMed
-
- Khoja L, Atenafu EG, Suciu S, Leyvraz S, Sato T, Marshall E, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol. 2019;30(8):1370–80. 10.1093/annonc/mdz176. - PubMed
-
- Nathan P, Hassel JC, Rutkowski P, Baurain J-F, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385(13):1196–206. 10.1056/NEJMoa2103485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

