Additional biomarkers and emm types associated with group A streptococcal toxic shock syndrome: a Japanese nationwide observational study
- PMID: 40193020
- PMCID: PMC12116948
- DOI: 10.1007/s10096-025-05116-6
Additional biomarkers and emm types associated with group A streptococcal toxic shock syndrome: a Japanese nationwide observational study
Abstract
Purpose: The incidence of invasive Group A Streptococcus (iGAS) infection and streptococcal toxic shock syndrome (STSS) is increasing. Early detection and diagnosis of cases that may progress to STSS are currently difficult. In this study, we aimed to identify biomarkers and emm type, one of the virulence factors, associated with STSS development.
Methods: In this multicentre observational study including patients with iGAS infection (n = 305), we investigated the relative associations of host factors, clinical manifestations, biomarkers, and emm type with STSS.
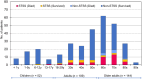
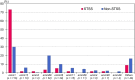
Results: The overall mortality rate was 15.4%; the fatality rate within 28 days of admission was higher in patients with STSS (67.9%, 38/56) than in those without (3.6%, 9/249). The most predominant type was emm1 (38%), detected in 73.2% of the patients with STSS. Risk factors for STSS identified by multivariable analysis included underlying kidney disease (odds ratio [OR], 10.7; 95% confidence interval [CI], 2.1-54.0, p = 0.004), bacteraemia without primary focus (OR, 3.6; 95% CI 1.2-11.1, p = 0.023), necrotizing fasciitis (OR, 8.7; 95% CI 2.6-29.4, p < 0.001), white blood cell count (WBC) < 4,000/µL (OR, 7.8; 95% CI 2.4-25.6, p = 0.001), serum creatine kinase (CK) ≥ 300 U/L (OR, 7.5; 95% CI 2.8-19.8, p < 0.001), and emm1 (OR, 5.2; 95% CI 2.0-13.4, p = 0.001).
Conclusion: WBC < 4,000/µL and CK level ≥ 300 U/L on admission are additional relevant biomarkers for STSS prediction. The most predominant iGAS type, emm1, was significantly associated with STSS.
Keywords: Emm type; Biomarker; Invasive group A Streptococcus infection; Streptococcal toxic shock syndrome.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was conducted in accordance with the Declaration of Helsinki and Guidelines for Epidemiologic Studies issued by the Japanese Ministry of Health, Labor, and Welfare. The study protocol was approved by the Institutional Review Board of the Keio University School of Medicine Ethics Committee (approval number: 20130455). Consent to participate: The need for written informed consent was waived owing to the use of anonymized stored samples and data. Patient medical records in the database were obtained anonymously using a survey form completed by the attending physicians. Consent to publish: Not applicable. Competing interests: The following authors received financial support within the last 3 years: K. U. from Pfizer Japan Inc., HORIBA, Ltd., and Abbott Diagnostics Medical Co., Ltd., J. S. from Roche Diagnostics K.K., Sysmex Corporation, Asahi Kasei Pharma Corporation, Shionogi & Co., Ltd., and Pfizer Japan Inc. All other authors declare that they have no conflicts of interest.
Figures

References
-
- Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Braunstein M, Rood JI (2019) Gram-positive pathogens, 3rd edn. American Society for Microbiology, Washington, DC. DOI. 10.1128/9781555819836
-
- Frost HR, Davies MR, Velusamy S et al (2020) Updated emm-typing protocol for Streptococcus pyogenes. Clin Microbiol Infect 26:946e5–946e8. 10.1016/j.cmi.2019.11.020 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

