Subthalamic Deep Brain Stimulation: Mapping Non-Motor Outcomes to Structural Connections
- PMID: 40193128
- PMCID: PMC11974458
- DOI: 10.1002/hbm.70207
Subthalamic Deep Brain Stimulation: Mapping Non-Motor Outcomes to Structural Connections
Abstract
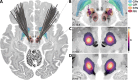
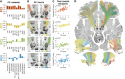
In Parkinson's Disease (PD), deep brain stimulation of the subthalamic nucleus (STN-DBS) reliably improves motor symptoms, and the circuits mediating these effects have largely been identified. However, non-motor outcomes are more variable, and it remains unclear which specific brain circuits need to be modulated or avoided to improve them. Since numerous non-motor symptoms potentially respond to DBS, it is challenging to independently identify the circuits mediating each one of them. Data compression algorithms such as principal component analysis (PCA) may provide a powerful alternative. This study aimed at providing a proof of concept for this approach by mapping changes along extensive score batteries to a few anatomical fiber bundles and, in turn, estimating changes in individual scores based on stimulation of these tracts. Retrospective data from 56 patients with PD and bilateral STN-DBS was included. The patients had undergone comprehensive clinical assessments covering changes in appetitive behaviors, mood, anxiety, impulsivity, cognition, and empathy. PCA was implemented to identify the main dimensions of neuropsychiatric and neuropsychological outcomes. Using DBS fiber filtering, we identified the structural connections whose stimulation was associated with change along these dimensions. Then, estimates of individual symptom outcomes were derived based on the stimulation of these connections by inverting the PCA. Finally, changes along a specific non-motor score were estimated in an independent validation dataset (N = 68) using the tract model. Four principal components were retained, which could be interpreted to reflect (i) general non-motor improvement; (ii) improvement of mood and cognition and worsening of trait impulsivity; (iii) improvement of cognition; and (iv) improvement of empathy and worsening of impulsive-compulsive behaviors. Each component was associated with the stimulation of spatially segregated fiber bundles connecting regions of the frontal cortex with the subthalamic nucleus. The extent of stimulation of these tracts was able to explain significant amounts of variance in outcomes for individual symptoms in the original cohort (circular analysis), as well as in the rank of depression outcomes in the independent validation cohort. Our approach represents an innovative concept for mapping changes along extensive score batteries to a few anatomical fiber bundles and could pave the way toward personalized deep brain stimulation.
Keywords: Parkinson's disease; deep brain stimulation; non‐motor symptoms; structural connectivity; subthalamic nucleus.
© 2025 The Author(s). Human Brain Mapping published by Wiley Periodicals LLC.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

