Digital tracking, provider decision support systems, and targeted client communication via mobile devices to improve primary health care
- PMID: 40193137
- PMCID: PMC11975193
- DOI: 10.1002/14651858.CD012925.pub2
Digital tracking, provider decision support systems, and targeted client communication via mobile devices to improve primary health care
Abstract
Background: Digital tracking on mobile devices, combined with clinical decision support systems and targeted client communication, can facilitate service delivery and potentially improve outcomes.
Objectives: To assess the effects of using a mobile device to track service use when combined with clinical decision support (Tracking + CDSS), with targeted client communications (Tracking + TCC), or both (Tracking + CDSS + TCC).
Search methods: Cochrane CENTRAL, MEDLINE, Embase, Ovid Population Information Online (POPLINE), K4Health and WHO Global Health Library (2000 to November 2022).
Selection criteria: Randomised and non-randomised trials in community/primary care settings.
Participants: primary care providers and clients Interventions: 1. Tracking + CDSS 2. Tracking + TCC 3. Tracking + CDSS + TCC Comparators: usual care (without digital tracking) DATA COLLECTION AND ANALYSIS: Two authors independently screened trials, extracted data and assessed risk of bias using the RoB 1 tool. We used a random-effects model to meta-analyse data producing risk differences (RD), risk ratios (RR), or odds ratios (OR) for dichotomous outcomes and mean differences (MD) for continuous outcomes. Evidence certainty was assessed using GRADE.
Main results: We identified 18 eligible studies (11 randomised, seven non-randomised) conducted in Bangladesh, China, Ethiopia, India, Kenya, Palestine, Uganda, and the USA. All non-randomised studies had a high risk of bias. These results are from randomised studies. 'Probably/may/uncertain' indicates 'moderate/low/very low' certainty evidence. Tracking + CDSS Relating to antenatal/ postnatal care: Providers' adherence to recommendations May slightly increase home visits in the week following delivery (2 studies, 4531 participants; RD 0.10 [0.07, 0.14]) May slightly increase counselling for initiating complementary feeding (2 studies, 4397 participants; RD 0.12 [0.08, 0.15]) May slightly increase the mean number of home visits in the month following delivery (1 study, 3023 participants; MD 0.75 [0.47, 1.03]) Uncertain effect on home visits within 24 hours of delivery Clients' health behaviours May slightly increase skin-to-skin care (1 study, 1544 participants; RD 0.05 [0.00, 0.10]) May slightly increase early breastfeeding (2 studies, 4540 participants; RD 0.08 [0.05, 0.12]) Uncertain effects on applying nothing to the umbilical cord, taking ≥ 90 iron-folate tablets during pregnancy, exclusively breastfeeding for six months, delaying the newborn's bath at least two days and Kangaroo Mother Care. Clients' health status May reduce low birthweight babies (1 study, 3023 participants; RR 0.53 [0.38, 0.73]) May increase infants with pneumonia or fever seeking care (1 study, 3470 participants; RR 1.13 [1.03, 1.24]) Uncertain effects on stillbirths, neonatal and infant deaths, or testing positive for HIV during antenatal testing Tracking + TCC Clients' health status In stroke patients over 12 months: May slightly increase blood pressure (BP) medication adherence (1 study, 1226 participants; RR 1.10 [1.00, 1.21]) May reduce deaths (1 study, 1226 participants; RR 0.52 [0.28, 0.96]) May slightly reduce systolic BP (1 study, 1226 participants; MD -2.80 mmHg [-4.90, -0.70]) May slightly improve EQ-5D scores (1 study, 1226 participants; MD 0.04 [0.02, 0.06]) May reduce stroke hospitalisations (1 study, 1226 participants; RR 0.45 [0.32, 0.64]). Tracking + CDSS + TCC Providers' adherence to recommendations Probably increases guideline adherence for antenatal screening and management of anaemia (1 study, 10,502 participants; OR 1.88 [1.52, 2.32]), diabetes (1 study, 8669 participants; OR 1.45 [1.14, 1.84}), hypertension (1 study, 15,555 participants; OR 1.62 [1.29, 2.04]) and probably leads to lower adherence for abnormal foetal growth (1 study, 1165 participants; OR 0.59 [0.37, 0.95]). May slightly increase nevirapine prophylaxis in infants of HIV+ve mothers (1 study, 609 participants; OR 1.75 [0.73, 4.19]) Data quality In pregnant women (1 study, 6367 participants), tracking + CDSS + TCC: Probably slightly reduces missing data for haemoglobin (RR 0.77 [0.71, 0.84]) but slightly more for BP at delivery (RR 1.16 [1.08, 1.24]) May have little or no effect on missing data on gestational age (RR 0.96 [0.81, 1.14]) or birthweight (RR 0.90 [0.77, 1.04]) Clients' health behaviour May have little or no effect on being on anti-retroviral therapy at delivery (1 study, 438 participants; OR 1.41 [0.81, 2.45]) or exclusive breastfeeding for six months (1 study, 695 participants; OR 1.74 [0.95, 3.17]) in HIV+ve mothers Uncertain effects on physical activity in high cardiovascular-risk adults Clients' health status May reduce the number of deaths in patients with hypertension and diabetes (1 study, 3698 participants; OR 0.61 [0.35, 1.06]) May reduce new cardiovascular events in high-cardiovascular risk adults over 6-18 months (1 study, 8642 participants; OR 0.58 [0.42, 0.80}) May slightly decrease in antenatal women severe hypertension, but the confidence interval includes both a decrease and increase (1 study, 6367 participants; OR 0.61 [0.27, 1.37]) In women receiving antenatal care (1 study, 6367 participants), tracking + CDSS + TCC maymake little or no difference to adverse pregnancy outcomes (OR 0.99 [0.87, 1.12]), moderate or severe anaemia (OR 0.82 [0.51, 1.31]), or large-for-gestational-age babies (OR 1.06 [0.90, 1.25]). In adults with hypertension or diabetes (1 study, 3324 participants), tracking + CDSS + TCC maymake little or no difference to HbA1c (MD 0.08 [-0.27, 0.43]), total cholesterol (MD -2.50 [-7.10, 2.10]), 10-year cardiovascular risk (MD -0.40 [-2.30, 1.50]), tobacco use (MD-0.05 [-0.47, 0.37]), alcohol use (MD 0.70 [-3.70, 5.10]), or PHQ-9 (MD -1.60 [-4.40, 1.20]). Uncertain effects on maternal or infant mortality before the baby reaches 18 months in HIV-positive mothers, patients who achieve optimal BP, BP controlled at five years, diastolic or systolic BP, body mass index, fasting glucose and quality of life in adults with hypertension or diabetes Client service utilisation May have little or no effect on missed early infant diagnosis visits (1 study, 1183 participants; OR 0.92 [0.63, 1.35]). Uncertain effects on linkage to care Client satisfaction Probably increases slightly the number of adults with hypertension or diabetes reporting "slightly/much better" change in the quality of care (1 study, 3324 participants; RR 1.02 [1.00, 1.03]). No studies evaluated time between presentation and appropriate management, timeliness of receiving/accessing care, provider acceptability/satisfaction, resource use, or unintended consequences.
Authors' conclusions: Digital tracking may improve primary care workers' ability to follow recommended antenatal and chronic disease practices, quality of patient records, patient health outcomes and service use. However, these interventions led to small or no outcome differences in most studies.
Copyright © 2025 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Smisha Agarwal: Bill and Melinda Gates Foundation (Grant / Contract), Digital Health Consulting (Independent Contractor ‐ Consultant), REACH (Fiduciary Officer).
Hanna Bergman: none known.
Weng Yee Chin: World Health Organization (Independent Contractor ‐ Other).
Marita Fønhus: none known. Former editorial staff at EPOC. MF was not involved in the editorial process of the review.
Hakan Safaralilo Foss: none known.
Tigest Tamrat: none known.
Claire Glenton: Norges Forskningsråd (Grant / Contract). Former editorial staff at EPOC. CG was not involved in the editorial process of the review.
Nicholas Henschke: none known.
Simon Lewin: World Health Organization (Norwegian Institute of Public Health: Grant / Contract), Cochrane Person‐Centred Care, Health Systems and Public Health Thematic Group (Fiduciary Officer). SL was previously a Co‐Coordinating Editor for Cochrane Effective Practice and Organisation of Care (EPOC) but was not involved in the editorial process of the review.
Garrett L Mehl: owns stock in Apple Computer.
Shivani Pandya: none known.
Natschja Ratanaprayul: none known.
Lavanya Vasudevan: World Health Organization (Independent Contractor—Consultant); LV is a co‐author of one of the included studies (Uddin 2016). The study was funded by a non‐profit organization, Grand Challenges Canada. LV was not involved in data extraction, assessment of the risk of bias, or certainty of the evidence for this study.
Figures

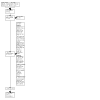

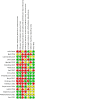






















Update of
- doi: 10.1002/14651858.CD012925
References
References to studies included in this review
Asiki 2018 {published data only}
-
- Asiki G, Newton R, Kibirige L, Kamali A, Marions L, Smedman L. Feasibility of using smartphones by village health workers for pregnancy registration and effectiveness of mobile phone text messages on reduction of homebirths in rural Uganda. PloS One 2018;27(13):e0198653. [DOI: 10.1371/journal.pone.0198653] - DOI - PMC - PubMed
Bull 2018 {published data only}
Carmichael 2019 {published data only}
-
- Borkum E, Sivasankaran A, Sridharan S, Rotz D, Sethi S, Manoranjini M, et al. Evaluation of the information and communication technology (ICT) continuum of care services (CCS) intervention in Bihar. https://mathematica.org/~/media/publications/pdfs/international/itc_ccs_... (accessed prior to 3 April 2025).
-
- Carmichael SL, Mehta K, Srikantiah S, Mahapatra T, Chaudhuri I, Balakrishnan R, et al. Use of mobile technology by frontline health workers to promote reproductive, maternal, newborn and child health and nutrition: a cluster randomized controlled trial in Bihar, India. Journal of Global Health 2019;9(2):0204249. [DOI: 10.7189/jogh.09.020424] - DOI - PMC - PubMed
Chen 2016 {published data only}
Hackett 2018 {published data only}
Ilozumba 2018 {published data only}
-
- Ilozumba O, Van Belle S, Dieleman M, Liem L, Choudhury M, Broerse JE. The effect of a community health worker utilized mobile health application on maternal health knowledge and behavior: a quasi-experimental study. Frontiers in Public Health 2018;6:133. [DOI: 10.3389/fpubh.2018.00133] - DOI - PMC - PubMed
Modi 2019 {published data only}
-
- Modi D, Desai S, Dave K, Shah S, Desai G, Dholakia N, et al. Cluster randomized trial of a mHealth intervention "ImTeCHO" to improve delivery of proven maternal, neonatal, and child care interventions through community-based Accredited Social Health Activists (ASHAs) by enhancing their motivation and strengthening supervision in tribal areas of Gujarat, India: study protocol for a randomized controlled trial. Trials 2017;18(1):270. [DOI: 10.1186/s13063-017-1998-0] - DOI - PMC - PubMed
-
- Modi D, Dholakia N, Gopalan R, Venkatraman S, Dave K, Shah S, et al. Mhealth intervention "ImTeCHO" to improve delivery of maternal, neonatal, and child care services - a cluster-randomized trial in tribal areas of Gujarat, India. PLoS Medicine 2019;16(10):e1002939. [DOI: 10.1371/journal.pmed.1002939] - DOI - PMC - PubMed
-
- Modi D, Saha S, Vaghela P, Dave K, Anand A, Desai S, et al. Costing and cost-effectiveness of a mobile health intervention (ImTeCHO) in improving infant mortality in tribal areas of Gujarat, India: cluster randomized controlled trial. JMIR MHealth and UHealth 2020;8(10):e17066. [DOI: 10.2196/17066] - DOI - PMC - PubMed
Patil 2022 {published data only}
-
- Patil SR, Nimmagadda S, Gopalakrishnan L, Avula R, Bajaj S, Diamond-Smith N, et al. Can digitally enabling community health and nutrition workers improve services delivery to pregnant women and mothers of infants? Quasi-experimental evidence from a national-scale nutrition programme in India. BMJ Global Health 2022;6 (Suppl 5):e007298. [DOI: 10.1136/bmjgh-2021-007298] - DOI - PMC - PubMed
Peiris 2019 {published data only}
-
- Peiris D, Praveen D, Mogulluru K, Ameer MA, Raghu A, Li Q, et al. SMARThealth India: a stepped-wedge, cluster-randomised controlled trial of a community health worker managed mobile health intervention for people assessed at high cardiovascular disease risk in rural India. PloS One 2019;14(3):e0213708. [DOI: 10.1371/journal.pone.0213708] - DOI - PMC - PubMed
-
- Praveen D, Patel A, McMahon S, Prabhakaran D, Clifford GD, Maulik PK, et al. A multifaceted strategy using mobile technology to assist rural primary healthcare doctors and frontline health workers in cardiovascular disease risk management: protocol for the SMARTHealthIndia cluster randomised controlled trial. Implementation Science 2013;8:137. - PMC - PubMed
Prabhakaran 2019 {published data only}
-
- Jha D, Gupta P, Ajay VS, Jindal D, Perel P, Prieto-Merino D, et al. Protocol for the mWellcare trial: a multicentre, cluster randomised, 12-month, controlled trial to compare the effectiveness of mWellcare, an mHealth system for an integrated management of patients with hypertension and diabetes, versus enhanced usual care in India. BMJ Open 2017;7(8):e014851. [DOI: 10.1136/bmjopen-2016-014851] - DOI - PMC - PubMed
-
- Prabhakaran D, Jha D, Prieto-Merino D, Roy A, Singh K, Ajay VS, et al. Effectiveness of an mHealth-based electronic decision support system for integrated management of chronic conditions in primary care: the mWellcare cluster-randomized controlled trial. Circulation 2019;139:380-91. [DOI: 10.1161/CIRCULATIONAHA.118.038192] - DOI - PubMed
Prinja 2017 {published data only}
-
- Prinja S, Nimesh R, Gupta A, Bahuguna P, Gupta M, Thakur JS. Impact of M-Health application used by community health volunteers on improving utilisation of maternal, new-born and child health care services in a rural area of Uttar Pradesh, India. Tropical Medicine & International Health 2017;22(7):895-907. [DOI: 10.1111/tmi.12895] - DOI - PubMed
-
- Prinja S, Nimesh R, Gupta A, Bahuguna P, Thakur JS, Gupta M, et al. Impact assessment and cost-effectiveness of M-Health application used by community health workers for maternal, newborn and child health care services in rural Uttar Pradesh, India: a study protocol. Global Health Action 2016;9:31473. [DOI: 10.3402/gha.v9.31473] - DOI - PMC - PubMed
Shiferaw 2016 {published data only}
-
- Shiferaw S, Spigt M, Tekie M, Abdullah M, Fantahun M, Dinant GJ. The effects of a locally developed mHealth intervention on delivery and postnatal care utilization: a prospective controlled evaluation among health centres in Ethiopia. PloS One 2016;11(7):e0158600. [DOI: 10.1371/journal.pone.0158600] - DOI - PMC - PubMed
Shiffman 2000 {published data only}
Suryavanshi 2020 {published data only}
-
- Suryavanshi N, Kadam A, Gupte N, Hegde A, Kanade S, Sivalenka S, et al. A mobile health-facilitated behavioural intervention for community health workers improves exclusive breastfeeding and early infant HIV diagnosis in India: a cluster randomized trial. Journal of the International AIDS Society 2020;23(7):e25555. [DOI: 10.1002/jia2.25555] - DOI - PMC - PubMed
-
- Suryavanshi N, Kadam A, Kanade S, Gupte N, Gupta A, Bollinger R, et al. Acceptability and feasibility of a behavioral and mobile health intervention (COMBIND) shown to increase uptake of prevention of mother to child transmission (PMTCT) care in India. BMC Public Health 2020;20(1):752. [DOI: 10.1186/s12889-020-08706-5] - DOI - PMC - PubMed
Uddin 2016 {published data only}
Vedanthan 2019 {published data only}
Venkateswaran 2022 {published data only}
-
- Venkateswaran M, Ghanem B, Abbas E, Khader KA, Ward IA, Awwad T, et al. A digital health registry with clinical decision support for improving quality of antenatal care in Palestine (eRegQual): a pragmatic, cluster-randomised, controlled, superiority trial. Lancet. Digital Health 2022;4(2):e126-36. [DOI: 10.1016/S2589-7500(21)00269-7] - DOI - PMC - PubMed
Yan 2021 {published data only}
-
- Yan LL, Gong E, Gu W, Turner EL, Gallis JA, Zhou Y, et al. Effectiveness of a primary care-based integrated mobile health intervention for stroke management in rural China (SINEMA): a cluster-randomized controlled trial. PLoS Medicine 2021;18(4):e1003582. [DOI: 10.1371/journal.pmed.1003582] - DOI - PMC - PubMed
References to studies excluded from this review
Abdel‐Kader 2011 {published data only}
Abidi 2018 {published data only}
-
- Abidi S, Vallis M, Piccinini-Vallis H, Imran SA, Abidi SS. Diabetes-related behavior change knowledge transfer to primary care practitioners and patients: implementation and evaluation of a digital health platform. JMIR Medical Informatics 2018;6(2):e25. [DOI: 10.2196/medinform.9629] - DOI - PMC - PubMed
Adams 2014 {published data only}
-
- Adams WG, Phillips BD, Bacic JD, Walsh KE, Shanahan CW, Paasche-Orlow MK. Automated conversation system before pediatric primary care visits: a randomized trial. Pediatrics 2014;134:e691-9. - PubMed
Adams 2016 {published data only}
-
- Adams AS, Bayliss EA, Schmittdiel JA, Altschuler A, Dyer W, Neugebauer R, et al. The Diabetes Telephone Study: design and challenges of a pragmatic cluster randomized trial to improve diabetic peripheral neuropathy treatment. Clinical Trials (London, England) 2016;13(3):286-93. [DOI: 10.1177/1740774516631530] - DOI - PMC - PubMed
Adjei 2015 {published data only}
-
- Adjei DN, Agyemang C, Dasah JB, Kuranchie P, Amoah AG. The effect of electronic reminders on risk management among diabetic patients in low resourced settings. Journal of Diabetes and Its Complications 2015;29:818-21. - PubMed
Andersson 2013 {published data only}
-
- Andersson ML, Bottiger Y, Lindh JD, Wettermark B, Eiermann B. Impact of the drug-drug interaction database SFINX on prevalence of potentially serious drug-drug interactions in primary health care. European Journal of Clinical Pharmacology 2013;69:565-71. - PubMed
Arbogast 2017 {published data only}
Atlas 2011 {published data only}
Atlas 2014 {published data only}
-
- Atlas SJ, Zai AH, Ashburner JM, Chang Y, Percac-Lima S, Levy DE, et al. Non-visit-based cancer screening using a novel population management system. Journal of the American Board of Family Medicine 2014;27:474-85. - PubMed
Atreja 2016 {published data only}
-
- Atreja A, Szigethy E, Colombel JF, Otobo E, Ullman T, Marion J, et al. Psychosocial burden among patients with IBD: prospectively collected data from 2 academic institutions. Inflammatory Bowel Diseases 2016;22:S29.
Baer 2015 {published data only}
Bailey 2016 {published data only}
-
- Bailey SC, Paasche-Orlow MK, Adams WG, Brokenshire SA, Hickson RP, Oramasionwu CU, et al. The electronic medication complete communication study: rationale and methods for a randomized controlled trial of a strategy to promote medication safety in ambulatory care. Contemporary Clinical Trials 2016;51:72-7. - PMC - PubMed
Bajaj 2016 {published data only}
Bell 2010 {published data only}
-
- Bell LM, Grundmeier R, Localio R, Zorc J, Fiks AG, Zhang X, et al. Electronic health record-based decision support to improve asthma care: a cluster-randomized trial. Pediatrics 2010;125:e770-7. - PubMed
Beratarrechea 2019 {published data only}
-
- Abrahams‐Gessel S, Beratarrechea A, Irazola V, Gutierrez L, Moyano D, Fernandez A, et al. Using mHealth tools to improve access, coverage and treatment of uninsured people with high cardiovascular disease risk in Argentina: a study protocol for a pragmatic cluster randomised trial. BMJ Innovations 2018;4:135-41. [DOI: 10.1136/bmjinnov-2017-000255] - DOI
-
- Beratarrechea A, Abrahams-Gessel S, Irazola V, Gutierrez L, Moyano D, Gaziano TA. Using mHealth tools to improve access and coverage of people with public health insurance and high cardiovascular disease risk in Argentina: a pragmatic cluster randomized trial. Journal of the American Heart Association 2019;8(8):e011799. [DOI: 10.1161/JAHA.118.011799] - DOI - PMC - PubMed
Biemba 2020 {published data only}
-
- Biemba G, Chiluba B, Yeboah-Antwi K, Silavwe V, Lunze K, Mwale RK, et al. Impact of mobile health-enhanced supportive supervision and supply chain management on appropriate integrated community case management of malaria, diarrhoea, and pneumonia in children 2-59 months: a cluster randomised trial in Eastern Province, Zambia. Journal of Global Health 2020;10(1):010425. [DOI: 10.7189/jogh.10.010425] - DOI - PMC - PubMed
Billah 2022 {published data only}
-
- Billah SM, Ferdous TE, Kelly P, Raynes-Greenow C, Siddique AB, Choudhury N, et al. Effect of nutrition counselling with a digital job aid on child dietary diversity: analysis of secondary outcomes from a cluster randomised controlled trial in rural Bangladesh. Maternal & Child Nutrition 2022;18(1):e13267. [DOI: 10.1111/mcn.13267] - DOI - PMC - PubMed
-
- Billah SM, Ferdous TE, Siddique AB, Raynes-Greenow C, Kelly P, Choudhury N, et al. The effect of electronic job aid assisted one-to-one counselling to support exclusive breastfeeding among 0-5-month-old infants in rural Bangladesh. Maternal & Child Nutrition 2022;18(3):e13377. [DOI: 10.1111/mcn.13377] - DOI - PMC - PubMed
Bloomfield 2005 {published data only}
-
- Bloomfield HE, Nelson DB, Ryn M, Neil BJ, Koets NJ, Basile JN, et al. A trial of education, prompts, and opinion leaders to improve prescription of lipid modifying therapy by primary care physicians for patients with ischemic heart disease. Quality & Safety in Health Care 2005;14:258-63. - PMC - PubMed
Bobrow 2016 {published data only}
-
- Bobrow K, Brennan T, Springer D, Levitt NS, Rayner B, Namane M, et al. Efficacy of a text messaging (SMS) based intervention for adults with hypertension: protocol for the StAR (SMS Text-message Adherence suppoRt trial) randomised controlled trial. BMC Public Health 2014;14:28. [DOI: 10.1186/1471-2458-14-28] - DOI - PMC - PubMed
-
- Bobrow K, Farmer AJ, Springer D, Shanyinde M, Yu LM, Brennan T, et al. Mobile phone text messages to support treatment adherence in adults with high blood pressure (SMS-Text Adherence Support [StAR]): a single-blind, randomized trial. Circulation 2016;133(6):592-600. [DOI: 10.1161/CIRCULATIONAHA.115.017530] - DOI - PMC - PubMed
Borbolla 2007 {published data only}
-
- Borbolla D, Giunta D, Figar S, Soriano M, Dawidowski A, Quiros FG. Effectiveness of a chronic disease surveillance system for blood pressure monitoring. Studies in Health Technology and Informatics 2007;129:223-7. - PubMed
Bourgeois 2010 {published data only}
-
- Bourgeois FC, Linder J, Johnson SA, Co JP, Fiskio J, Ferris TG. Impact of a computerized template on antibiotic prescribing for acute respiratory infections in children and adolescents. Clinical Pediatrics 2010;49:976-83. - PubMed
Bowman 2015 {published data only}
-
- Bowman S, Butz A, Rothman R, Anders J, Johnson B, Trent M. Unmet need for HIV screening among adolescents with pelvic inflammatory disease. Journal of Adolescent Health 2015;56:S23-4.
Castillo 2019 {published data only}
-
- Castillo M, Alexander N, Rubiano L, Rojas C, Navarro A, Rincon D, et al. Randomized trial evaluating an mHealth intervention for the early community-based detection and follow-up of cutaneous leishmaniasis in rural Colombia. PLoS Neglected Tropical Diseases 2023;17(3):e0011180. [DOI: 10.1371/journal.pntd.0011180] - DOI - PMC - PubMed
Dregan 2014 {published data only}
-
- Dregan A, Van Staa TP, McDermott L, McCann G, Ashworth M, Charlton J, et al. Point-of-care cluster randomized trial in stroke secondary prevention using electronic health records. Stroke 2014;45:2066-71. - PubMed
Feldstein 2006a {published data only}
-
- Feldstein A, Elmer PJ, Smith DH, Herson M, Orwoll E, Chen C, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. Journal of the American Geriatrics Society 2006;54:450-7. - PubMed
Feldstein 2006b {published data only}
-
- Feldstein AC, Smith DH, Perrin N, Yang X, Rix M, Raebel MA, et al. Improved therapeutic monitoring with several interventions: a randomized trial. Archives of Internal Medicine 2006;166:1848-54. - PubMed
Fiks 2009 {published data only}
-
- Fiks AG, Hunter KF, Localio AR, Grundmeier RW, Bryant-Stephens T, Luberti AA, et al. Impact of electronic health record-based alerts on influenza vaccination for children with asthma. Pediatrics 2009;124:159-69. - PubMed
Fiks 2013 {published data only}
Forrest 2013 {published data only}
-
- Forrest CB, Fiks AG, Bailey LC, Localio R, Grundmeier RW, Richards T, et al. Improving adherence to otitis media guidelines with clinical decision support and physician feedback. Pediatrics 2013;131:e1071-81. - PubMed
Fricton 2011 {published data only}
-
- Fricton J, Rindal DB, Rush W, Flottemesch T, Vazquez G, Thoele MJ, et al. The effect of electronic health records on the use of clinical care guidelines for patients with medically complex conditions. Journal of the American Dental Association 2011;142:1133-42. - PubMed
Gill 2011 {published data only}
Gill 2012 {published data only}
-
- Gill JM, Chen YX, Grimes A, Klinkman MS. Using electronic health record-based tools to screen for bipolar disorder in primary care patients with depression. Journal of the American Board of Family Medicine 2012;25:283-90. - PubMed
Grant 2015 {published data only}
-
- Grant RW, Ashburner JM, Jernigan MC, Chang J, Borowsky LH, Chang Y, et al. Randomized trial of a health IT tool to support between-visit-based laboratory monitoring for chronic disease medication prescriptions. Journal of General Internal Medicine 2015;30(5):619-25. [DOI: 10.1007/s11606-014-3152-y] - DOI - PMC - PubMed
Gupta 2014 {published data only}
-
- Gupta A, Gholami P, Turakhia MP, Friday K, Heidenreich PA. Clinical reminders to providers of patients with reduced left ventricular ejection fraction increase defibrillator referral: a randomized trial. Circulation. Heart Failure 2014;7:140-5. - PubMed
Holbrook 2011 {published data only}
-
- Holbrook A, Pullenayegum E, Thabane L, Troyan S, Foster G, Keshavjee K, et al. Shared electronic vascular risk decision support in primary care: computerization of Medical Practices for the Enhancement of Therapeutic Effectiveness (COMPETE III) randomized trial. Archives of Internal Medicine 2011;171:1736-44. - PubMed
Hsu 2013 {published data only}
Lim 2011 {published data only}
Lim 2016 {published data only}
-
- Lim S, Kang SM, Kim KM, Moon JH, Choi SH, Hwang H, et al. Multifactorial intervention in diabetes care using real-time monitoring and tailored feedback in type 2 diabetes. Acta Diabetologica 2016;53:189-98. - PubMed
Lim 2019 {published data only}
-
- Lim S, Wyatt LC, Mammen S, Zanowiak JM, Mohaimin S, Goldfeld KS, et al. The DREAM Initiative: study protocol for a randomized controlled trial testing an integrated electronic health record and community health worker intervention to promote weight loss among South Asian patients at risk for diabetes. Trials 2019;20(1):635. - PMC - PubMed
Lo 2007 {published data only}
-
- Lo HG, Matheny ME, Seger DL, Bates DW, Gandhi TK. Non-interruptive drug-lab alerts in ambulatory care. In: AMIA Annual Symposium Proceedings. AMIA Symposium. Vol. 1. 2007:1038. [PMID: ] - PubMed
Lokman 2015 {published data only}
-
- Lokman S, Volker D, Zijlstra-Vlasveld M, Smit F, Feltz-Cornelis C. Return-to-work intervention versus care as usual for sick listed employees with common mental disorders: trial-based economic evaluation shows promise. Journal of Mental Health Policy and Economics 2015;18:S26-7.
Luo 2019 {published data only}
Mann 2012 {published data only}
-
- Mann D, Kannry J, Wisnivesky JP, Stulman J, McCullagh L, Sofianou A, et al. Electronic health record tool reduces antibiotic use: the integrated clinical prediction rules (ICPR) trial. Journal of General Internal Medicine 2012;27:S181.
Martins 2017 {published data only}
McGinn 2013 {published data only}
-
- McGinn TG, McCullagh L, Kannry J, Knaus M, Sofianou A, Wisnivesky JP, et al. Efficacy of an evidence-based clinical decision support in primary care practices: a randomized clinical trial. JAMA Internal Medicine 2013;173:1584-91. - PubMed
McKinstry 2013 {published data only}
McNabb 2015 {published data only}
-
- McNabb M, Chukwu E, Ojo O, Shekhar N, Gill CJ, Salami H, et al. Assessment of the quality of antenatal care services provided by health workers using a mobile phone decision support application in northern Nigeria: a pre/post-intervention study. PloS One 2015;10(5):e0123940. [DOI: 10.1371/journal.pone.0123940] - DOI - PMC - PubMed
Mekonnen 2019 (excl) {published data only}
-
- Mekonnen ZA, Tilahun B, Alemu K, Were M. Effect of mobile phone text message reminders on improving completeness and timeliness of routine childhood vaccinations in North-West, Ethiopia: a study protocol for randomised controlled trial. BMJ Open 2019;9(11):e031254. [DOI: 10.1136/bmjopen-2019-031254] - DOI - PMC - PubMed
Miloh 2016 {published data only}
O'Connor 2011 {published data only}
Orrell 2015 {published data only}
-
- Orrell C, Cohen K, Mauff K, Bangsberg DR, Maartens G, Wood R. A randomized controlled trial of real-time electronic adherence monitoring with text message dosing reminders in people starting first-line antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes 2015;70(5):495-502. [DOI: 10.1097/QAI.0000000000000770] - DOI - PubMed
Park 2012 {published data only}
-
- Park MJ, Kim HS. Evaluation of mobile phone and Internet intervention on waist circumference and blood pressure in post-menopausal women with abdominal obesity. International Journal of Medical Informatics 2012;81:388-94. - PubMed
Quinn 2009 {published data only}
-
- Quinn CC, Gruber-Baldini AL, Shardell M, Weed K, Clough SS, Peeples M, et al. Mobile diabetes intervention study: testing a personalized treatment/behavioral communication intervention for blood glucose control. Contemporary Clinical Trials 2009;30:334-46. - PubMed
Quinn 2012 {published data only}
-
- Quinn CC, Gruber-Baldini AL, Shardell MD, Terrin ML. A cluster-randomized trial of a mobile phone behavioral intervention for blood glucose control: primary and secondary outcomes. Journal of Diabetes Science and Technology 2012;6:A156-7.
Quinn 2014 {published data only}
Redfern 2020 {published data only}
-
- Redfern J, Usherwood T, Coorey G, Mulley J, Scaria A, Neubeck L, et al. A consumer-direct digital health intervention for cardiovascular risk management in primary care: the Consumer Navigation of Electronic Cardiovascular Tools (CONNECT) randomised controlled trial. European Heart Journal 2019;40(Supplement 1):ehz746.0278. [ACTRN: ACTRN12613000715774]
Robbins 2012 {published data only}
Santero 2018 {published data only}
Sarrasst 2021 {published data only}
-
- Bakibinga P, Kamande E, Omuya M, Ziraba AK, Kyobutungi C. The role of a decision-support smartphone application in enhancing community health volunteers' effectiveness to improve maternal and newborn outcomes in Nairobi, Kenya: quasi-experimental research protocol. BMJ Open 2017;7(7):e014896. [DOI: 10.1136/bmjopen-2016-014896] - DOI - PMC - PubMed
-
- Sarrassat S, Lewis JJ, Some AS, Somda S, Cousens S, Blanchet K. An Integrated eDiagnosis Approach (IeDA) versus standard IMCI for assessing and managing childhood illness in Burkina Faso: a stepped-wedge cluster randomised trial. BMC Health Services Research 2021;21(1):354. [DOI: 10.1186/s12913-021-06317-3] - DOI - PMC - PubMed
Sequist 2005 {published data only}
Sequist 2012 {published data only}
Shah 2012 {published data only}
-
- Shah S, Singh K, Ali MK, Mohan V, Kadir MM, Unnikrishnan AG, et al. Improving diabetes care: multi-component cardiovascular disease risk reduction strategies for people with diabetes in South Asia - the CARRS multi-center translation trial. Diabetes Research and Clinical Practice 2012;98:285-94. - PMC - PubMed
Shaikh 2015 {published data only}
-
- Shaikh U, Berrong J, Nettiksimmons J, Byrd RS. Impact of electronic health record clinical decision support on the management of pediatric obesity. American Journal of Medical Quality 2015;30:72-80. - PubMed
Shelley 2011 {published data only}
-
- Shelley D, Tseng TY, Matthews AG, Wu D, Ferrari P, Cohen A, et al. Technology-driven intervention to improve hypertension outcomes in community health centers. American Journal of Managed Care 2011;17:SP103-10. - PubMed
Shrestha 2019 {published data only}
-
- Shrestha SS, Bhavnani S, Casaclang-Verzosa G, Khalil M, Thamman R, Patel J, et al. Improving the efficiency of healthcare delivery with digital health technologies in the ASE Foundation Community Health Outreach Imaging and Cardiovascular Examinations (CHOICE) Program: a cluster randomized trial. Journal of the American Society of Echocardiography 2019;32(6):B111.
Silveira 2019 {published data only}
-
- Silveira DV, Marcolino MS, Machado EL, Ferreira CG, Alkmim MB, Resende ES, et al. Development and evaluation of a mobile decision support system for hypertension management in the primary care setting in Brazil: mixed-methods field study on usability, feasibility, and utility. JMIR MHealth and UHealth 2019;7(3):e9869. - PMC - PubMed
Singh 2020 {published data only}
-
- Singh JK, Acharya D, Paudel R, Gautam S, Adhikari M, Kushwaha SP, et al. Effects of female community health volunteer capacity building and text messaging intervention on gestational weight gain and hemoglobin change among pregnant women in Southern Nepal: a cluster randomized controlled trial. Frontiers in Public Health 2020;8:312. - PMC - PubMed
Sumayya 2021 {published data only}
-
- Sumayya MB. Efficacy of Electronic Mobile Phone Application Compared with Mother and Child Health Booklet in Improving Quality of Antenatal Care from First Visit through Third Trimester at Kenyatta National Hospital, a Randomized Controlled Trial [Doctoral Dissertation]. Nairobi: University of Nairobi, 2021. [PAN AFRICAN CLINICAL TRIALS REGISTRY: PACTR202001700173081]
Szilagyi 2015 {published data only}
Tajmir 2017 {published data only}
Tamblyn 2010 {published data only}
-
- Tamblyn R, Reidel K, Huang A, Taylor L, Winslade N, Bartlett G, et al. Increasing the detection and response to adherence problems with cardiovascular medication in primary care through computerized drug management systems: a randomized controlled trial. Medical Decision Making 2010;30:176-88. - PubMed
Tang 2012 {published data only}
Taveras 2013 {published data only}
-
- Taveras EM, Marshall R, Horan CM, Gillman MW, Hacker K, Kleinman KP, et al. Rationale and design of the STAR randomized controlled trial to accelerate adoption of childhood obesity comparative effectiveness research. Contemporary Clinical Trials 2013;34:101-8. - PubMed
Taveras 2017 {published data only}
Tian 2015 {published data only}
Vollmer 2014 {published data only}
Weingart 2013 {published data only}
-
- Weingart SN, Carbo A, Tess A, Chiappetta L, Tutkus S, Morway L, et al. Using a patient internet portal to prevent adverse drug events: a randomized, controlled trial. Journal of Patient Safety 2013;9:169-75. - PubMed
Westgard 2019 {published data only}
Wu 2015 {published data only}
Zurovac 2011 {published data only}
Zurovac 2012 {published data only}
References to ongoing studies
Bassi 2022 {published data only}
-
- Bassi A, Arfin S, John O, Praveen D, Arora V, Kalra OP, et al. Innovative mobile-health led participatory approach to comprehensive screening and treatment of diabetes (IMPACT diabetes): rationale, design, and baseline characteristics. International Journal of Diabetes in Developing Countries 2023;43:352-62. [DOI: 10.1007/s13410-022-01082-3] - DOI
Bates 2018 {published data only}
-
- Bates LA, Hicks JP, Walley J, Robinson E. Evaluating the impact of Marie Stopes International's digital family planning counselling application on the uptake of long-acting and permanent methods of contraception in Vietnam and Ethiopia: a study protocol for a multi-country cluster randomised controlled trial. Trials 2018;19(1):420. [DOI: 10.1186/s13063-018-2815-0] - DOI - PMC - PubMed
Blanchet 2016 {published data only}
-
- Blanchet K, Lewis JJ, Pozo-Martin F, Satouro A, Somda S, Ilboudo P, et al. A mixed methods protocol to evaluate the effect and cost-effectiveness of an Integrated electronic Diagnosis Approach (IeDA) for the management of childhood illnesses at primary health facilities in Burkina Faso. Implementation Science 2016;11:111. - PMC - PubMed
CTRI/2019/12/022435 {published data only}
-
- CTRI/2019/12/022435. Development and impact of a healthcare decision support system on treatment outcomes of diabetes and hypertension. https://ctri.nic.in/Clinicaltrials/showallp.php?mid1=37564&EncHid=&a... (first registered 18 December 2019).
Gong 2019 {published data only}
-
- Gong E, Gu W, Sun C, Turner EL, Zhou Y, Li Z, et al. System-integrated technology-enabled model of care to improve the health of stroke patients in rural China: protocol for SINEMA-a cluster-randomized controlled trial. American Heart Journal 2020;207:27-39. - PubMed
Green 2015 {published data only}
-
- Green EP, Catalani C, Diero L, Carter EJ, Gardner A, Ndwiga C, et al. Do clinical decision-support reminders for medical providers improve isoniazid preventative therapy prescription rates among HIV-positive adults? Study protocol for a randomized controlled trial. Trials 2015;16:141. [DOI: 10.1186/s13063-015-0558-8] - DOI - PMC - PubMed
-
- NCT01934309. Do clinical decision-support reminders for medical providers the prevalence of IPT initiation among HIV positive adults in Western Kenya? https://clinicaltrials.gov/show/NCT01934309 (first posted 4 September 2013).
Lejone 2020 {published data only}
-
- Lejone TI, Kopo M, Bachmann N, Brown JA, Glass TR, Muhairwe J, et al. PEBRA trial - effect of a peer-educator coordinated preference-based ART service delivery model on viral suppression among adolescents and young adults living with HIV: protocol of a cluster-randomized clinical trial in rural Lesotho. BMC Public Health 2020;20(1):425. - PMC - PubMed
Lygidakis 2019 {published data only}
-
- Lygidakis C, Uwizihiwe JP, Kallestrup P, Bia M, Condo J, Vögele C. Community- and mHealth-based integrated management of diabetes in primary healthcare in Rwanda (D²Rwanda): the protocol of a mixed-methods study including a cluster randomised controlled trial. BMJ Open 2019;9(7):e028427. [DOI: 10.1136/bmjopen-2018-028427] - DOI - PMC - PubMed
-
- NCT03376607. Community- and mHealth-based integrated management of diabetes in primary healthcare in Rwanda (D²Rwanda). https://clinicaltrials.gov/ct2/show/NCT03376607 (first posted 18 December 2017).
Morkrid 2021 {published data only}
-
- Morkrid K, Bogale B, Abbas E, Abu Khader K, Abu Ward I, Attalh A, et al. ERegCom-quality improvement dashboard for healthcare providers and targeted client communication to pregnant women using data from an electronic health registry to improve attendance and quality of antenatal care: study protocol for a multi-arm cluster randomized trial. Trials 2021;22(1):47. [DOI: 10.1186/s13063-020-04980-1] - DOI - PMC - PubMed
Nagraj 2023 {published data only}
-
- Nagraj S, Kennedy SH, Jha V, Norton R, Hinton L, Billot L, et al. SMARThealth Pregnancy: feasibility and acceptability of a complex intervention for high-risk pregnant women in rural India: protocol for a pilot cluster randomised controlled trial. Frontiers in Global Women's Health 2021;2:620759. [DOI: 10.3389/fgwh.2021.620759] - DOI - PMC - PubMed
NCT02909179 {published data only}
-
- NCT02909179. Measuring the impact of a mobile health system to support healthy pregnancies and improve newborn survival (mCARE-II). https://clinicaltrials.gov/ct2/show/NCT02909179 (first posted 21 September 2016).
NCT03189004 {published data only}
-
- NCT03189004. Assessing the impact of mobile phone technology to improve Health Nutrition and Population (HNP) service utilization in rural Bangladesh through pilot intervention. https://clinicaltrials.gov/ct2/show/NCT03189004 (first posted 16 June 2017).
NCT05511701 {published data only}
-
- NCT05511701. Preventing ischemic heart disease with mHealth (mobile health), electronic decision support and community health workers (PRIMECare). https://clinicaltrials.gov/study/NCT05511701 (first posted 23 August 2022). [CLINICALTRIALS.GOV ID: NCT05511701]
Peiris 2016 {published data only}
Velen 2022 {published data only}
-
- Velen K, Nguyen VN, Nguyen BH, Dang T, Nguyen HA, Vu DH, et al. Harnessing new mHealth technologies to strengthen the management of multidrug-resistant tuberculosis in Vietnam (V-SMART trial): a protocol for a randomised controlled trial. BMJ Open 2022;12(6):e052633. [DOI: 10.1136/bmjopen-2021-052633] - DOI - PMC - PubMed
Venkateshmurthy 2018 {published data only}
-
- NCT03164317. To test the effectiveness of a trained nurse led, m-health enabled intervention to control blood pressure in India. https://clinicaltrials.gov/study/NCT03164317 (first posted 23 May 2017). [CLINICALTRIALS.GOV: NCT03164317]
-
- Srinivasapura Venkateshmurthy N, Ajay VS, Mohan S, Jindal D, Anand S, Kondal D, et al. M-power heart project - a nurse care coordinator led, mHealth enabled intervention to improve the management of hypertension in India: study protocol for a cluster randomized trial. Trials 2018;19(1):429. [DOI: 10.1186/s13063-018-2813-2] - DOI - PMC - PubMed
Additional references
Agarwal 2016
-
- Agarwal S, LeFevre AE, Lee J, L'Engle K, Mehl G, Sinha C, et al. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ (Clinical Research Ed.) 2016;352:i1174. - PubMed
Agarwal 2021
-
- Agarwal S, Glenton C, Tamrat T, Henschke N, Maayan N, Fønhus MS, et al. Decision-support tools via mobile devices to improve quality of care in primary healthcare settings. Cochrane Database of Systematic Reviews 2021, Issue 7. Art. No: CD012944. [DOI: 10.1002/14651858.CD012944.pub2] - DOI - PMC - PubMed
Ames 2019
-
- Ames HM, Glenton C, Lewin S, Tamrat T, Akama E, Leon N. Clients' perceptions and experiences of targeted digital communication accessible via mobile devices for reproductive, maternal, newborn, child, and adolescent health: a qualitative evidence synthesis. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD013447. [DOI: 10.1002/14651858.CD013447] - DOI - PMC - PubMed
Arain 2010
Atasoy 2019
-
- Atasoy H, Greenwood BN, McCullough JS. The digitization of patient care: a review of the effects of electronic health records on health care quality and utilization. Annual Review of Public Health 2019;40:487-500. - PubMed
Awofeso 2004
-
- Awofeso N. What is the difference between ’primary care’ and ’primary healthcare’? Quality in Primary Care 2004;12:93-4.
Blank 2013
-
- Blank A, Prytherch H, Kaltschmidt J, Krings A, Sukums F, Mensah N, et al. Quality of prenatal and maternal care: bridging the know-do gap (QUALMAT study): an electronic clinical decision support system for rural Sub-Saharan Africa. BMC Medical Informatics and Decision-Making 2013;13(1):4. - PMC - PubMed
Bright 2012
-
- Bright TJ, Wong A, Dhurjati R, Bristow E, Bastian L, Coeytaux RR, et al. Effect of clinical decision-support systems: a systematic review. Annals of Internal Medicine 2012;157(1):29-43. - PubMed
Covidence [Computer program]
-
- Covidence. Version accessed prior to 16 December 2024. Melbourne, Australia: Veritas Health Innovation, 2024. Available at https://www.covidence.org.
CRDM 2024
-
- Public Health Informatics Institute. Collaborative requirements development methodology. https://phii.org/crdm/ (accessed 5 March 2024).
Das 2018
De Cock 2020
Divall 2013
-
- Divall P, Camosso-Stefinovic J, Baker R. The use of personal digital assistants in clinical decision making by health care professionals: a systematic review. Health Informatics Journal 2013;19(1):16-28. - PubMed
Dtree International 2017
-
- Dtree International. Safer deliveries. http://www.d-tree.org/saving-lives/womens-lives/safer-deliveries/ (accessed 26 December 2017).
EPOC 2017a
-
- Cochrane Effective Practice and Organisation of Care (EPOC). Data collection form. EPOC resources for review authors, 2017. Available at https://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 2 November 2017).
EPOC 2017b
-
- Cochrane Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors, 2017. Available at https://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 2 November 2017).
EPOC 2017c
-
- Cochrane Effective Practice and Organisation of Care (EPOC). Interrupted time series (ITS) analyses. EPOC resources for review authors, 2017. Available at https://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 2 November 2017).
EPOC 2017d
-
- Cochrane Effective Practice and Organisation of Care (EPOC). Analysis in EPOC reviews. EPOC resources for review authors, 2017. Available at https://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 2 November 2017).
EPOC 2017e
-
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC worksheets for preparing a 'Summary of findings' table using GRADE. EPOC resources for review authors, 2017. Available at https://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 2 November 2017).
EPOC 2017f
-
- Effective Practice and Organisation of Care (EPOC). Reporting the effects of an intervention in EPOC reviews. EPOC resources for review authors, 2017. Available at https://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 5 November 2017).
Free 2013
Frøen 2016
Global Health Watch 2011
-
- Global Health Watch. Primary health care: a review and critical appraisal of its revitalization. www.ghwatch.org/sites/www.ghwatch.org/files/B1_0.pdf (accessed 1 October 2017).
GRADEpro GDT 2024 [Computer program]
-
- GRADEpro GDT. Version accessed prior to 16 December 2024. Hamilton (ON): McMaster University (developed by Evidence Prime), 2024. Available at https://www.gradepro.org.
Gurol‐Urganci 2013
Guyatt 2008
Haazen 2015
-
- Haazen DS, Slote A, Al-Shorbaji N, D’Adamo M. EHealth technical paper for MA4Health - measurement and accountability for results in health: a common agenda for the post 2015 era. http://www.searo.who.int/entity/health_situation_trends/the-roadmap-for-... (accessed 1 October 2017).
Higgins 2024
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated August 2024). Available from www.training.cochrane.org/handbook 2024.
International Telecommunications Union 2015
-
- International Telecommunications Union. ICT facts and figures 2015. https://www.itu.int/en/ITU-D/Statistics/Documents/facts/ICTFactsFigures2... (accessed 1 October 2017).
Kahn 2009
-
- Kahn JS, Aulakh V, Bosworth A. What it takes: characteristics of the ideal personal health record. Health Affairs 2009;28 (2):369-76. - PubMed
Kawamoto 2005
Kirkham 2017
Lund 2012
Mohanan 2015
Moja 2014
Muldoon 2006
Odendaal 2020
-
- Odendaal WA, Anstey Watkins J, Leon N, Goudge J, Griffiths F, Tomlinson M, et al. Health workers' perceptions and experiences of using mHealth technologies to deliver primary healthcare services: a qualitative evidence synthesis. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD011942. [DOI: 10.1002/14651858.CD011942.pub2] - DOI - PMC - PubMed
Orton 2018
Page 2021
Palmer 2020a
Palmer 2020b
-
- Palmer MJ, Henschke N, Bergman H, Villanueva G, Maayan N, Tamrat T, et al. Targeted client communication via mobile devices for improving maternal, neonatal, and child health. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013679. [DOI: 10.1002/14651858.CD013679] - DOI - PMC - PubMed
RevMan 2025 [Computer program]
-
- Review Manager (RevMan). Version 8.14.0. The Cochrane Collaboration, 2025. Available at https://revman.cochrane.org.
Sterne 2011
Sutton 2020
Triantafyllidis 2020
-
- Triantafyllidis A, Polychronidou E, Alexiadis A, Rocha CL, Oliveira DN, Da Silva AS, et al. Computerized decision support and machine learning applications for the prevention and treatment of childhood obesity: a systematic review of the literature. Artificial Intelligence in Medicine 2020;104:101844. - PubMed
Uddin 2016
Vodopivec‐Jamsek 2012
Whittaker 2016
WHO 2008
-
- World Health Organization. Primary health care: now more than ever; 2008. http://www.who.int/whr/2008/en/ (accessed 10 October 2017).
WHO 2016
-
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: World Health Organization, 2016. [PMID: ] - PubMed
WHO 2019
-
- World Health Organization. WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening. Geneva: World Health Organization, 2019. [ISBN-13: 978-92-4-155050-5] - PubMed
WHO 2020
-
- World Health Organization. Digital Implementation Investment Guide: Integrating Digital Interventions into Health Programmes. Geneva: World Health Organization, 2020. [LICENCE: CC BY-NC-SA 3.0 IGO.]
WHO 2024
-
- World Health Organization. Handbook for Digitizing Primary Health Care: Optimizing Person Centred Decision-Support Point of Service Solutions. Geneva: World Health Organization, 2024. [LICENCE: CC BY-NC-SA 3.0 IGO]
WHO/ITU 2012
-
- World Health Organization, International Telecommunications Union. National eHealth Strategy Toolkit; 2012. https://www.itu.int/dms_pub/itu-d/opb/str/D-STR-E_HEALTH.05-2012-PDF-E.pdf (accessed 1 October 2017).
References to other published versions of this review
Argawal 2018
-
- Agarwal S, Vasudevan L, Tamrat T, Glenton C, Lewin S, Bergman H, et al. Digital tracking, provider decision support systems, and targeted client communication via mobile devices to improve primary health care. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD012925. [DOI: 10.1002/14651858.CD012925] - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

