N6-methyladenosine in DNA promotes genome stability
- PMID: 40193195
- PMCID: PMC11975372
- DOI: 10.7554/eLife.101626
N6-methyladenosine in DNA promotes genome stability
Abstract
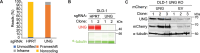
DNA base lesions, such as incorporation of uracil into DNA or base mismatches, can be mutagenic and toxic to replicating cells. To discover factors in repair of genomic uracil, we performed a CRISPR knockout screen in the presence of floxuridine, a chemotherapeutic agent that incorporates uracil and fluorouracil into DNA. We identified known factors, such as uracil DNA N-glycosylase (UNG), and unknown factors, such as the N6-adenosine methyltransferase, METTL3, as required to overcome floxuridine-driven cytotoxicity. Visualized with immunofluorescence, the product of METTL3 activity, N6-methyladenosine, formed nuclear foci in cells treated with floxuridine. The observed N6-methyladenosine was embedded in DNA, called 6mA, and these results were confirmed using an orthogonal approach, liquid chromatography coupled to tandem mass spectrometry. METTL3 and 6mA were required for repair of lesions driven by additional base-damaging agents, including raltitrexed, gemcitabine, and hydroxyurea. Our results establish a role for METTL3 and 6mA in promoting genome stability in mammalian cells, especially in response to base damage.
Keywords: 6mA; METTL3; MMR; N6-methyladenosine; U-BER; UNG; cancer biology; cell biology; human.
Plain language summary
All mammals store their genetic material in the form of DNA, which is constantly damaged by factors such as ultraviolet radiation, chemicals, and errors during cellular processes. To prevent such damage from causing harmful mutations, it is important that cells have repair mechanisms that can fix damaged DNA. Some drugs used to treat cancer cause damage to DNA by incorporating uracil, a compound that doesn’t belong in DNA. This can lead to DNA mutations if not repaired. An enzyme known as UNG2 is involved in repairing this damage by removing the uracil-based lesions. However, the process of uracil repair was not fully understood. To investigate, Conti et al. treated cancer cells with the drug floxuridine, which is known to cause uracil-based DNA damage. A genetic screening technique identified that a gene encoding an enzyme known as METTL3 is required for repairing uracil-related damage. Further experiments suggested that METTL3 adds markers known as m6A to DNA to help direct repair by UNG2. Inhibiting METTL3 made the cells more sensitive to the drug treatment and reduced the amount of UNG2 at sites of DNA damage. While m6A marks are known to exist in bacterial DNA, evidence of them in mammalian DNA has been a topic of debate. The findings of Conti et al. suggest that these modifications form in response to DNA damage and help to facilitate repair DNA in mammalian cells. Further research is needed to clarify how METTL3 and m6A marks interact with other DNA repair pathways. Gaining a greater understanding of these repair processes could help future research into strategies to treat diseases driven by DNA damage, such as cancer.
© 2024, Conti et al.
Conflict of interest statement
BC, QX, LS, DS, MO previous employee of Pfizer and owns Pfizer stock, LN, DT previous employee of Pfizer, SV, TM, PS, JA current employee of Pfizer and owns Pfizer stock, CN current employee of Pfizer, ND No competing interests declared, RV, PP, BS P.P., R.T.V., N.D.L.C., and B.R.S., receivedresearch funding from Pfizer Inc
Figures









Update of
- doi: 10.1101/2024.07.20.604187
- doi: 10.7554/eLife.101626.1
- doi: 10.7554/eLife.101626.2
References
-
- Christenson ES, Gizzi A, Cui J, Egleston M, Seamon KJ, DePasquale M, Orris B, Park BH, Stivers JT. Inhibition of human uracil dna glycosylase sensitizes a large fraction of colorectal cancer cells to 5-fluorodeoxyuridine and raltitrexed but not fluorouracil. Molecular Pharmacology. 2021;99:412–425. doi: 10.1124/molpharm.120.000191. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

