Amino acids in cancer: Understanding metabolic plasticity and divergence for better therapeutic approaches
- PMID: 40193251
- PMCID: PMC12038367
- DOI: 10.1016/j.celrep.2025.115529
Amino acids in cancer: Understanding metabolic plasticity and divergence for better therapeutic approaches
Abstract
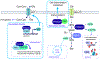
Metabolic reprogramming is a hallmark of malignant transformation. While initial studies in the field of cancer metabolism focused on central carbon metabolism, the field has expanded to metabolism beyond glucose and glutamine and uncovered the important role of amino acids in tumorigenesis and tumor immunity as energy sources, signaling molecules, and precursors for (epi)genetic modification. As a result of the development and application of new technologies, a multifaceted picture has emerged, showing that context-dependent heterogeneity in amino acid metabolism exists between tumors and even within distinct regions of solid tumors. Understanding the complexity and flexibility of amino acid metabolism in cancer is critical because it can influence therapeutic responses and predict clinical outcomes. This overview discusses the current findings on the heterogeneity in amino acid metabolism in cancer and how understanding the metabolic diversity of amino acids can be translated into more clinically relevant therapeutic interventions.
Keywords: CP: Cancer; CP: Metabolism; amino acids; cancer metabolism; metabolic heterogeneity.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Cancer Genome Atlas Research Network; Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, Davis C, Wheeler DA, Murray BA, and Schmidt L (2016). Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N. Engl. J. Med 374, 135–145. 10.1056/NEJMoa1505917. - DOI - PMC - PubMed
-
- Morotti M, Bridges E, Valli A, Choudhry H, Sheldon H, Wigfield S, Gray N, Zois CE, Grimm F, Jones D, et al. (2019). Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc. Natl. Acad. Sci. USA 116, 12452–12461. 10.1073/pnas.1818521116. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

