Expedient Assembly of Multiantennary N-Glycans from Common N-Glycan Cores with Orthogonal Protection for the Profiling of Glycan-Binding Proteins
- PMID: 40193327
- PMCID: PMC12006998
- DOI: 10.1021/jacs.5c02356
Expedient Assembly of Multiantennary N-Glycans from Common N-Glycan Cores with Orthogonal Protection for the Profiling of Glycan-Binding Proteins
Abstract
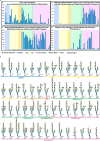
Complex-type N-glycans are structurally diverse molecules, responsible for many biological processes, yet the specific sequences of N-glycans involved in biological recognition remain largely unknown. Despite the recent development of many efficient chemoenzymatic approaches, it is still lacking a general approach to produce structurally diverse complex-type N-glycans. Here, we designed two common precursors equipped with orthogonal protecting groups for antennary differentiation and selective glycan elongation. The N-acetyllactosamine (LacNAc) repeat modules were synthesized separately based on iterative Au(I) promoted glycosylation and programmable one-pot strategy and were incorporated into the N-glycan core structure in a site-specific manner. The final removal of benzyl groups was cleanly achieved using pressurized flow chemistry. A total of 51 N-glycans were assembled and presented as an array to study the binding specificity toward a panel of influenza hemagglutinins and other lectins. The established method allows a rapid and previously infeasible synthesis of asymmetric bi- and triantennary N-glycans, especially with the LacNAc repeats residing at a specific arm, bringing in new opportunities to study carbohydrate-receptor interactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Shriver Z.; Raguram S.; Sasisekharan R. Glycomics: a pathway to a class of new and improved therapeutics. Nat. Rev. Drug Discovery 2004, 3, 863.10.1038/nrd1521. - DOI - PubMed
- Gong Y.; Qin S.; Dai L.; Tian Z. The glycosylation in SARS-CoV-2 and its receptor ACE2. Signal Transduct. Targeted Ther. 2021, 6, 396.10.1038/s41392-021-00809-8. - DOI - PMC - PubMed
- Cao L.; Diedrich J. K.; Kulp D. W.; Pauthner M.; He L.; Park S. K. R.; Sok D.; Su C. Y.; Delahunty C. M.; Menis S.; Andrabi R.; Guenaga J.; Georgeson E.; Kubitz M.; Adachi Y.; Burton D. R.; Schief W. R.; Yates III J. R.; Paulson J. C. Global site-specific N-glycosylation analysis of HIV envelope glycoprotein. Nat. Commun. 2017, 8, 14954.10.1038/ncomms14954. - DOI - PMC - PubMed
-
- Wallace E. N.; West C. A.; McDowell C. T.; Lu X.; Bruner E.; Mehta A. S.; Aoki-Kinoshita K. F.; Angel P. M.; Drake R. R. An N-glycome tissue atlas of 15 human normal and cancer tissue types determined by MALDI-imaging mass spectrometry. Sci. Rep. 2024, 14, 489.10.1038/s41598-023-50957-w. - DOI - PMC - PubMed
- Turiák L.; Shao C.; Meng L.; Khatri K.; Leymarie N.; Wang Q.; Pantazopoulos H.; Leon D. R.; Zaia J. Workflow for combined proteomics and glycomics profiling from histological tissues. Anal. Chem. 2014, 86, 9670.10.1021/ac5022216. - DOI - PMC - PubMed
-
- Wang Z.; Chinoy Z. S.; Ambre S. G.; Peng W.; Mcbride R.; de Vries R. P.; Glushka J.; Paulson J. C.; Boons G.-J. A general Strategy for the Chemoenzymatic Synthesis of Asymmetrically Branched N-glycans. Science 2013, 341, 379.10.1126/science.1236231. - DOI - PMC - PubMed
- Gagarinov I. A.; Li T.; Toraño J. S.; Caval T.; Srivastava A. D.; Kruijtzer J. A. W.; Heck A. J. R.; Boons G.-J. Chemoenzymatic Approach for the Preparation of Asymmetric Bi-, Tri-, and Tetra-Antennary N-Glycans from a Common Precursor. J. Am. Chem. Soc. 2017, 139, 1011.10.1021/jacs.6b12080. - DOI - PMC - PubMed
-
- Liu L.; Prudden A. R.; Capicciotti C. J.; Bosman G. P.; Yang J.-Y.; Chapla D. G.; Moremen K. W.; Boons G.-J. Streamlining the Chemoenzymatic Synthesis of Complex N-glycans by a Stop and Go Strategy. Nat. Chem. 2019, 11, 161.10.1038/s41557-018-0188-3. - DOI - PMC - PubMed
- Ma S.; Liu L.; Eggink D.; Herfst S.; Fouchier R. A. M.; de Vries R. P.; Boons G.-J. Asymmetrical Biantennary Glycans Prepared by a Stop-and-Go Strategy Reveal Receptor Binding Evolution of Human Influenza A Viruses. JACS Au 2024, 4, 607.10.1021/jacsau.3c00695. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

