In utero therapy for spinal muscular atrophy: closer to clinical translation
- PMID: 40193572
- PMCID: PMC12404777
- DOI: 10.1093/brain/awaf123
In utero therapy for spinal muscular atrophy: closer to clinical translation
Abstract
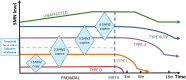
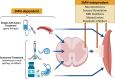
5q-Spinal muscular atrophy (SMA) has been a trailblazer in the development of advanced therapies for inherited diseases. SMA is an autosomal recessive disorder affecting mainly motor neurons in the anterior horn of the spinal cord and brainstem motor nucle but currently considered a systemic disease. Advances in understanding the genetics of SMA led to the development of disease-modifying therapies, either transferring a healthy version of SMN1, the causative gene absent or altered in SMA, or modulating SMN2, a highly homologous but less functional version of SMN1, present in all patients. After successful clinical trials, these approaches have resulted in three marketed therapies. Severe SMA, 'type I', is the most common type and is considered both a developmental arrest and neurodegenerative disorder. As pathology starts during fetal life in type I patients, a cure is unlikely even when treatment is started shortly after birth in the pre- or mildly symptomatic state. In utero fetal therapy offers the opportunity to mitigate further or possibly prevent manifestations of the disease. This review discusses clinical and developmental aspects of SMA, the advanced therapies approved (gene therapy, antisense oligonucleotide and small molecule compounds), and the rationale, options and challenges, including ethical and safety issues, to initiate in utero therapy. Looking beyond sporadic case reports of prenatal intervention, clinical trials of in utero SMA therapy can be envisaged and should be carefully designed and evaluated to move closer to clinical translation.
Keywords: in-utero therapy; fetus; gene therapy; human development; pre-symptomatic; spinal muscular atrophy.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
E.F.T. received personal compensation for consultancy from Novartis Gene Therapies, Inc., Biogen, Biologix, Cytokinetics, Novartis, and Roche, and research funding from Biogen/Ionis and Roche. RSF has received personal compensation for advisory board/data safety monitoring board participation from Novartis Gene Therapies, Inc., Biogen, Catabasis, Capricor, ReveraGen, Roche, and Scholar Rock; editorial fees from Elsevier for co-editing a neurology textbook; license fees from the Children's Hospital of Philadelphia; research funding from Novartis Gene Therapies, Inc., Biogen, Capricor, Catabasis, ReveraGen, Roche, and Scholar Rock; and received personal compensation for serving as a speaker for a workshop with the National Academy of Sciences. E.C. and R.J.Y-M. have filed a patent application for a novel sequence-optimized SMN1 transgene. G.L. has no conflicts of interest to disclose.
Figures

References
-
- Ogino S, Wilson RB. Genetic testing and risk assessment for spinal muscular atrophy (SMA). Hum Genet. 2002;111:477–500. - PubMed
-
- Calucho M, Bernal S, Alías L, et al. Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord. 2018;28:208–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

