A monoclonal anti-hemagglutinin stem antibody modified with zanamivir protects against both influenza A and B viruses
- PMID: 40193611
- PMCID: PMC12012527
- DOI: 10.1073/pnas.2424889122
A monoclonal anti-hemagglutinin stem antibody modified with zanamivir protects against both influenza A and B viruses
Abstract
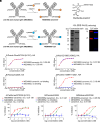
Influenza remains a significant public health threat. Both monoclonal antibodies and small-molecule inhibitors can target the influenza surface glycoproteins hemagglutinin (HA) or neuraminidase (NA) for prevention and treatment of influenza. Here, we combine the strengths of anti-influenza antibodies and small molecules by site-specific conjugation of the NA inhibitor zanamivir to MEDI8852, an HA-specific fully human monoclonal antibody. MEDI8852 targets the conserved stem region of HA and inhibits HA-mediated fusion of the viral and host cell membranes. Elimination of virus-infected cells involves Fcγ receptor-mediated effector functions. The efficacy of MEDI8852 is limited to influenza A viruses. Zanamivir, on the other hand, binds to the active site of NA in both influenza A and B viruses to inhibit NA activity and virus release. However, because of its small size, zanamivir has a short half-life and requires repeated dosing at high concentrations. We produced a MEDI8852-zanamivir antibody-drug conjugate (ADC) that engages Fc-mediated effector functions and benefits from neonatal Fc receptor (FcRn)-mediated recycling. The MEDI8852-zanamivir conjugate extends the circulatory half-life of zanamivir, targets both influenza HA and NA, and shows enhanced antibody-dependent cellular cytotoxicity (ADCC) compared to MEDI8852 alone. The MEDI8852-zanamivir conjugate protected mice from a lethal (10 × LD50) challenge with influenza A and B viruses at a dose similar to that required for broadly neutralizing anti-NA antibodies, with the added advantage of simultaneously targeting NA (influenza A and B) and HA (influenza A).
Keywords: anti-hemagglutinin monoclonal antibody; antibody–drug conjugate; antiviral therapy; influenza; neuraminidase inhibitor.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- World Health Organization, Influenza (Seasonal) Fact Sheet. (2023) (https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)).
-
- Yamayoshi S., Kawaoka Y., Current and future influenza vaccines. Nat. Med. 25, 212–220 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

