Project EVOLVE: an international analysis of postimmunotherapy lineage switch, an emergent form of relapse in leukemia
- PMID: 40193715
- PMCID: PMC12333221
- DOI: 10.1182/blood.2024026655
Project EVOLVE: an international analysis of postimmunotherapy lineage switch, an emergent form of relapse in leukemia
Abstract
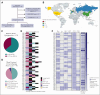
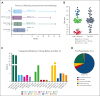
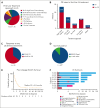
Lineage switch (LS), defined as the immunophenotypic transformation of acute leukemia, has emerged as a mechanism of relapse after antigen-targeted immunotherapy, which is associated with dismal outcomes. Through an international collaborative effort, we identified cases of LS after a host of antigen-targeted therapies (eg, CD19, CD22, CD38, and CD7), described how LS was diagnosed, reviewed treatment approaches, and analyzed overall outcomes for this form of postimmunotherapy relapse. Collectively, 75 cases of LS were evaluated, including 53 (70.7%) cases of B-cell acute lymphoblastic leukemia (B-ALL) transforming to acute myeloid leukemia (AML), 17 (22.7%) cases of B-ALL transforming to mixed phenotypic acute leukemia (MPAL)/acute leukemias of ambiguous lineage (ALAL), and 5 (6.7%) cases of rare LS presentation (ie, T-cell ALL to AML). An additional 10 cases with incomplete changes in immunophenotype, referred to as "lineage drift" were also described. With a primary focus on the 70 cases of LS from B-ALL to AML or MPAL/ALAL, LS emerged at a median of 1.5 months (range, 0-36.5) after immunotherapy, with 81.4% presenting with LS within the first 6 months from the most proximal immunotherapy. Although most involved KMT2A rearrangements (n = 45, 64.3%), other rare cytogenetic and/or molecular alterations were uniquely observed. Treatment outcomes were generally poor, with remission rates of <40%. The median overall survival after LS diagnosis was 4.8 months. Outcomes were similarly poor for those with rare immunophenotypes of LS or lineage drift. This global initiative robustly categorizes lineage changes after immunotherapy and, through enhanced understanding, establishes a foundation for improving outcomes of LS.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution.
Conflict of interest statement
Conflict-of-interest disclosure: H.A.-A. has served on advisory boards for Adaptive, Vertex, and Johnson & Johnson; and received study support from Adaptive. B.H.C. has received research funding from Deliver Therapeutics. D.S.D. has sponsored research with Syndax; is a consultant for Tempus, Amgen, and Y-mAbs Therapeutics; and serves on the advisory board of Day One Bio. F.E.C. is a consultant with SPD Oncology, Amgen, CTI BioPharma, AbbVie, MorphoSys, Association of Community Cancer Centers, PharmaEssentia, Bristol Myers Squibb, Geron, Sobi, and DAVA Oncology; received clinical trial grant support (principal investigator) to the University of Virginia from Amgen, Celgene, SPD Oncology, Sanofi, Bristol Myers Squibb, FibroGen, PharmaEssentia, BioSight, MEI Pharma, Novartis, and Arog Pharmaceuticals; and received travel grant support from DAVA Oncology. S.A.G. receives clinical research funding from Novartis, Cellectis, Kite, Vertex, and Servier; consults for Novartis, Eureka, and Adaptive; and has advised Novartis, Adaptimmune, Vertex, Allogene, Jazz Pharmaceuticals, and Cabaletta. E.M.H. is a consultant for Novartis. A.A.M. reports research funding from Bristol Myers Squibb, Stemline, Gilead, Incyte, and Novartis, paid to institution. K.R. served as consultant (from April 2023 to April 2024) for Sumitomo Pharma Inc for presenting on patient experience and training clinical research coordinators. A. Stevens reports research funding from AbbVie Pharmaceuticals and Gilead Pharmaceuticals. S.R. reports honoraria and/or travel support from Novartis, Servier, Celgene/Bristol Myers Squibb, Kite/Gilead, Pfizer, Clinigen, and Amgen; and reports being part of data and safety monitoring board in a clinical trial sponsored by Novartis, and of a data monitoring committee in a clinical trial sponsored by Autolus. I.A. served on an advisory board for Kite, Jazz, Syndax, Takeda, Wugen, Pfizer, and Adaptive; and reports research support from MacroGenic, AbbVie, and Jazz. N.B. reports honoraria from Amgen, Pfizer, Novartis, and Gilead. S.R.R. served as consultant on the data and safety monitoring committee for Pfizer, and steering committee for AbbVie. S.G. reports honoraria/speaker fees from Novartis and Autolus; reports patents with University College London Business and Autolus Ltd; and serves on a trial steering committee for Autolus Ltd. N.N.S. receives research funding from Lentigen, Vor Bio, and CARGO therapeutics; and has participated on advisory boards (no honoraria) for Sobi, Allogene, invoX, ImmunoACT, and Vor Bio. The remaining authors declare no competing financial interests.
Figures


Comment in
-
Postimmunotherapy lineage switch: where to from here?Blood. 2025 Jul 24;146(4):400-402. doi: 10.1182/blood.2025029260. Blood. 2025. PMID: 40705391 No abstract available.
References
-
- Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8(10):1219–1226. - PubMed
-
- Kurzer JH, Weinberg OK. To B- or not to B-: a review of lineage switched acute leukemia. Int J Lab Hematol. 2022;44(suppl 1):64–70. - PubMed
-
- Rossi JG, Bernasconi AR, Alonso CN, et al. Lineage switch in childhood acute leukemia: an unusual event with poor outcome. Am J Hematol. 2012;87(9):890–897. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

