[Research progress on collagen secretion mechanisms in scarring]
- PMID: 40194913
- PMCID: PMC12062945
- DOI: 10.3724/zdxbyxb-2024-0535
[Research progress on collagen secretion mechanisms in scarring]
Abstract
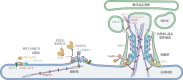
Scar formation is characterized by dynamic alterations in collagen secretion, which critically determine scar morphology and pathological progression. In fibroblasts, collagen secretion is initiated through the activation of cytokine- and integrin-mediated signaling pathways, which promote collagen gene transcription. The procollagen polypeptide α chains undergo extensive post-translational modifications, including hydroxylation and glycosylation, within the endoplasmic reticulum (ER), followed by folding and assembly into triple-helical procollagen. Subsequent intracellular trafficking involves the sequential transport of procollagen through the ER, Golgi apparatus, and plasma membrane, accompanied by further structural refinements prior to extracellular secretion. Once secreted, procollagen is enzymatically processed to form mature collagen fibrils, which drive scar tissue remodeling. Recent advances in elucidating regulation of collagen secretion have identified pivotal molecular targets, such as transforming growth factor-beta 1 (TGF-β1), prolyl 4-hydroxylase (P4H), heat shock protein 47 (HSP47), and transport and Golgi organization protein 1 (TANGO1), providing novel therapeutic strategies to mitigate pathological scar hyperplasia and improve regenerative outcomes. This review provides a comprehensive analysis of the molecular mechanisms governing collagen secretion during scar formation, with emphasis on signaling cascades, procollagen biosynthesis, intracellular transport dynamics, and post-translational modifications, thereby offering a framework for developing targeted anti-scar therapies.
瘢痕形成过程中,胶原分泌的动态变化直接影响瘢痕的形态特征和病理进展。成纤维细胞的胶原分泌过程是通过启动细胞因子和整合素介导信号通路的激活,进而诱导胶原基因转录。随后,前胶原多肽α链在内质网中合成,并经历精细的翻译后修饰、蛋白质折叠和组装后形成前胶原。前胶原通过内质网、高尔基体和质膜的逐级转运和修饰,最终分泌至细胞外,促进胶原纤维的合成。对胶原分泌过程及其调控机制的深入研究揭示了转化生长因子β1、脯氨酸-4-羟化酶、热休克蛋白47、转运和高尔基组织蛋白1等多个潜在治疗靶点,为瘢痕异常增生的干预及组织再生提供了新的策略。本文综述了瘢痕形成过程中胶原分泌的关键机制,重点介绍了信号通路激活、前胶原合成、转运及修饰的分子机制,以期为未来的抗瘢痕治疗提供参考。.
Keywords: Collagen secretion; Molecular mechanism; Review; Scar.
Conflict of interest statement
所有作者均声明不存在利益冲突
The authors declare that there is no conflict of interests
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous