Tspo Depletion Exacerbates Steatosis Through Fatty Acid Uptake
- PMID: 40195072
- PMCID: PMC11975627
- DOI: 10.1111/jcmm.70500
Tspo Depletion Exacerbates Steatosis Through Fatty Acid Uptake
Abstract
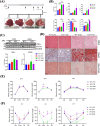
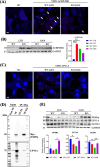
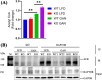
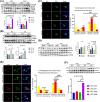
Previous studies demonstrated that Tspo loss causes simple steatosis (SS) in hepatocytes in vitro. However, its effect on SS in vivo remains unclear. In this study, we hypothesise that Tspo loss promotes early-stage MASLD. WT and Tspo KO rats were fed a Gubra Amylin NASH (GAN) diet for 8 weeks to induce SS. Tspo KO rats fed the GAN diet (KO GAN) exhibited increased insulin resistance, higher plasma cholesterol, and elevated hepatic triacylglycerol (TAG) levels, along with higher de novo lipogenesis (DNL) and free fatty acid (FFA) uptake, evidenced by increased fatty acid synthase (FASN) and CD36 expression. The Acyl-coenzyme A binding protein/diazepam-binding inhibitor-TSPO complex facilitated FA transport to the mitochondria, where carnitine palmitoyltransferase 1A (CPT1A) directed them for β-oxidation. TSPO interacted with CPT1A in the outer mitochondrial membrane, while its depletion increased CPT1A expression, boosting FA oxidation. Primary Tspo KO rat hepatocytes and stably overexpressed CD36 (CD36_OE) in Huh7 cells displayed impaired mitochondrial function and compromised mitochondrial membrane potential. KO GAN livers had significantly elevated AcCoA, which acetylated RAPTOR, activating mTORC1 to suppress autophagy. Overall, Tspo deficiency exacerbates the advancement of SS by enhancing CD36-mediated FFA uptake, elevating AcCoA levels, compromising mitochondrial function and impairing autophagy during the early stages of MASLD.
Keywords: MASLD; RAPTOR; acetyl‐coenzyme a; de novo lipogenesis; fatty acid oxidation.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Lazarus J. V., Mark H. E., Anstee Q. M., et al., “Advancing the Global Public Health Agenda for NAFLD: A Consensus Statement,” Nature Reviews. Gastroenterology & Hepatology 19 (2022): 60–78. - PubMed
-
- Harrison S. A., Bedossa P., Guy C. D., et al., “A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH With Liver Fibrosis,” New England Journal of Medicine 390 (2024): 497–509. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

