The SIRT1 activator SRT2104 exerts exercise mimetic effects and promotes Duchenne muscular dystrophy recovery
- PMID: 40195304
- PMCID: PMC11977210
- DOI: 10.1038/s41419-025-07595-z
The SIRT1 activator SRT2104 exerts exercise mimetic effects and promotes Duchenne muscular dystrophy recovery
Abstract
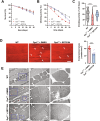
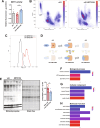
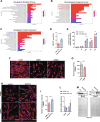
Duchenne muscular dystrophy (DMD) is a devastating genetic disorder, whose management is still a major challenge, despite progress in genetic and pharmacological disease-modifying treatments have been made. Mitochondrial dysfunctions contribute to DMD, however, there are no effective mitochondrial therapies for DMD. SIRT1 is a NAD+-dependent deacetylase that controls several key processes and whose impairment is involved in determining mitochondrial dysfunction in DMD. In addition to well-known resveratrol, other potent selective activators of SIRT1 exist, with better pharmacokinetics properties and a safer profile. Among these, SRT2104 is the most promising and advanced in clinical studies. Here we unveil the beneficial effects of SRT2104 in flies, mice, and patient-derived myoblasts as different models of DMD, demonstrating an anti-inflammatory, anti-fibrotic, and pro-regenerative action of the drug. We elucidate, by molecular dynamics simulations, that a conformational selection mechanism is responsible for the activation of SIRT1. Further, the impact of SRT2104 in reshaping muscle proteome and acetylome profiles has been investigated, highlighting effects that mimic those induced by exercise. Overall, our data suggest SRT2104 as a possible therapeutic candidate to successfully counteract DMD progression.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no conflicts of interest. Ethical approval: All procedures of animal experiments comply with ethical standards and were approved by the Italian Ministry of Health (approval no. 761/2022-PR). The two pediatric patient-derived immortalized cell lines were granted by biobank MyoLine and used according to the MTA with Prof. Mouly, director of MyoLine biobank in the frame of the agreement with Professor Ornella Cappellari and Professor Matteo Giovarelli respectively at the University of Bari and Milan.
Figures


References
-
- Emery AE. Population frequencies of inherited neuromuscular diseases-a world survey. Neuromuscul Disord. 1991;1:19–29. - PubMed
-
- Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscular Disord. 2014;24:482–91. - PubMed
MeSH terms
Substances
Grants and funding
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 24077/AFM-Téléthon (French Muscular Dystrophy Association)
- 24077/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 24077/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 20568/AFM-Téléthon (French Muscular Dystrophy Association)
- 2017FJSM9S/Ministero della Salute (Ministry of Health, Italy)
- 2020ELYA32004/Ministero della Salute (Ministry of Health, Italy)
- 2017FJSM9S/Ministero della Salute (Ministry of Health, Italy)
- 2020ELYA32004/Ministero della Salute (Ministry of Health, Italy)
LinkOut - more resources
Full Text Sources

