Pre-fertilization-origin preservation of brown fat-mediated energy expenditure in humans
- PMID: 40195445
- PMCID: PMC12021649
- DOI: 10.1038/s42255-025-01249-2
Pre-fertilization-origin preservation of brown fat-mediated energy expenditure in humans
Abstract
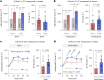
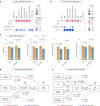
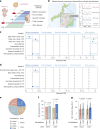
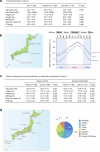
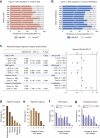
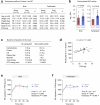
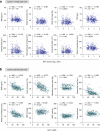
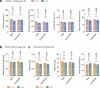
Environmental thermal stress substantially affects cellular plasticity of thermogenic adipocytes and energy balance through transcriptional and epigenetic mechanisms in rodents. However, roles of cold-adaptive epigenetic regulation of brown adipose tissue (BAT) in systemic energy metabolism in humans remained poorly understood. Here we report that individuals whose mothers conceived during cold seasons exhibit higher BAT activity, adaptive thermogenesis, increased daily total energy expenditure and lower body mass index and visceral fat accumulation. Structural equation modelling indicated that conception during the cold season protects against age-associated increase in body mass index through BAT activation in offspring. Meteorological analysis revealed that lower outdoor temperatures and greater fluctuations in daily temperatures during the fertilization period are key determinants of BAT activity. These findings suggest that BAT metabolic fate and susceptibility of metabolic diseases are preprogrammed by the epigenetic inheritance of cold exposure before the fertilization in humans.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
MeSH terms
Grants and funding
- JP20gm1310007/Japan Agency for Medical Research and Development (AMED)
- JP16H06390, JP20H04835, JP20K21747, JP21H04826, JP22K18411/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP21K08548, JP20K22647/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP22590227, JP18K11013/MEXT | Japan Society for the Promotion of Science (JSPS)
- the SECOM Science and Technology Foundation/Secom Science and Technology Foundation (SSTF)
LinkOut - more resources
Full Text Sources

