A vaccine chatbot intervention for parents to improve HPV vaccination uptake among middle school girls: a cluster randomized trial
- PMID: 40195450
- PMCID: PMC12176647
- DOI: 10.1038/s41591-025-03618-6
A vaccine chatbot intervention for parents to improve HPV vaccination uptake among middle school girls: a cluster randomized trial
Abstract
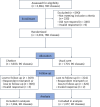
Conversational artificial intelligence, in the form of chatbots powered by large language models, offers a new approach to facilitating human-like interactions, yet its efficacy in enhancing vaccination uptake remains under-investigated. This study assesses the effectiveness of a vaccine chatbot in improving human papillomavirus (HPV) vaccination among female middle school students aged 12-15 years across diverse socioeconomic settings in China, where HPV vaccination is primarily paid out-of-pocket. A school-based cluster randomized trial was conducted from 18 January to 31 May 2024. The study included 2,671 parents from 180 middle school classes stratified by socioeconomic setting, school and grade level in Shanghai megacity, and urban and rural regions of Anhui Province. Participants were randomly assigned to either the intervention group (90 classes, 1,294 parents), which engaged with the chatbot for two weeks, or the control group (90 classes, 1,377 parents), which received usual care. The primary outcome was the receipt or scheduled appointment of the HPV vaccine for participants' daughters. In intention-to-treat analyses, 7.1% of the intervention group met this outcome versus 1.8% of the control group (P < 0.001) over a two-week intervention period. In addition, there was a statistically significant increase in HPV vaccination-specific consultations with health professionals (49.1% versus 17.6%, P < 0.001), along with enhanced vaccine literacy (P < 0.001) and rumor discernment (P < 0.001) among participants using the chatbot. These findings indicate that the chatbot effectively increased vaccination and improved parental vaccine literacy, although further research is necessary to scale and sustain these gains. Clinical trial registration: NCT06227689 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- The Global Cancer Observatory. Cervix uteri. Fact sheet; https://gco.iarc.who.int/media/globocan/factsheets/cancers/23-cervix-ute... (2022).
-
- The Global Cancer Observatory. China. Fact sheet; https://gco.iarc.who.int/media/globocan/factsheets/populations/160-china... (2022).
-
- Yuan, M., Zhao, X., Wang, H., Hu, S. & Zhao, F. Trend in cervical cancer incidence and mortality rates in China, 2006–2030: a Bayesian age-period-cohort modeling study. Cancer Epidemiol. Biomarkers Prev.32, 825–833 (2023). - PubMed
-
- Duan, R. et al. Temporal trends and projection of cancer attributable to human papillomavirus infection in China, 2007–2030. Cancer Epidemiol. Biomarkers Prev.31, 1130–1136 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

