The butyrate derived from probiotic Clostridium butyricum exhibits an inhibitory effect on multiple myeloma through cell death induction
- PMID: 40195469
- PMCID: PMC11976985
- DOI: 10.1038/s41598-025-97038-8
The butyrate derived from probiotic Clostridium butyricum exhibits an inhibitory effect on multiple myeloma through cell death induction
Abstract
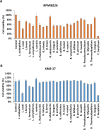
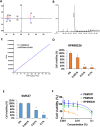
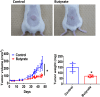
Multiple myeloma (MM) is a hematological malignancy characterized by a poor prognosis. While certain probiotics have been shown to produce antitumor molecules that inhibit solid tumor progression, it remains unclear whether probiotic-derived compounds can exert similar effects on hematological tumors, such as MM. In this study, we screened the cell-free culture supernatants (CFCS) of 24 probiotic strains for antitumor effects against multiple myeloma (MM) cells and identified that the CFCS from Clostridium butyricum (C. butyricum) demonstrated the most significant reduction in MM cell viability. Further fractionation of this CFCS through reverse-phase and gel filtration chromatography revealed a high enrichment of butyrate in the antitumor fraction, as confirmed by gas chromatography-mass spectrometry. Butyrate reduced MM cell viability in a concentration-dependent manner. Butyrate was significantly more cytotoxic to RPMI-8226 cells than peripheral blood mononuclear cells (PBMCs) isolated from two non-cancerous individuals. In the xenograft model of RPMI-8226 cells, butyrate showed significant inhibition of tumor formation. Cell cycle analysis showed that butyrate induced G1 phase arrest and increased sub-G1 phase, suggesting DNA fragmentation. Western blot analysis demonstrated that butyrate treatment led to cleaved poly ADP-ribose polymerase (PARP) accumulation. Additionally, flow cytometry showed an increase in annexin V positive MM cells, indicating apoptosis. Butyrate also exhibited synergistic antitumor activity when combined with bortezomib, a proteasome inhibitor. These findings suggest that probiotic-derived molecules, including butyrate, may enhance the therapeutic effect of hematological malignancy, such as MM.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Hiroaki Konishi and Mikihiro Fujiya report that Kamui Pharma Inc. provides financial support. They are part of a joint research department funded by Kamui Pharma Inc. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Ethics statement: All experiments were performed according to the Guidelines of the Public Health Service Policy on the Humane Use and Care of Laboratory Animals. The study received ethical approval for using an opt-out methodology from the Medical Ethics Committee of Asahikawa Medical University (Approval No. R6-005). All animal experiments were reported in accordance with the ARRIVE guidelines.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

