Dexamethasone: a double-edged sword in the treatment of osteoarthritis
- PMID: 40195473
- PMCID: PMC11976973
- DOI: 10.1038/s41598-025-96050-2
Dexamethasone: a double-edged sword in the treatment of osteoarthritis
Abstract
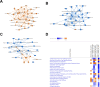
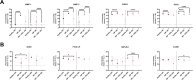
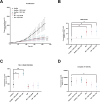
Glucocorticoids are widely used to manage osteoarthritis (OA) symptoms, but long-term safety concerns exist. This study investigates the therapeutic potential of dexamethasone (DEX) and triamcinolone acetonide (TA) in chondrocytes, evaluating their anti-inflammatory effects and potential detrimental actions. This study evaluated the effects of DEX and TA on the expression of pro-inflammatory genes in inflamed chondrocytes. In addition, the effects of DEX treatment on chondrocytes were analyzed using next-generation sequencing, high-resolution mass spectrometry, proliferation and metabolic rate, wound healing capacity and senescence-associated B-galactosidase assays. A single therapeutic dose of DEX (40nM) effectively reduced the expression of inflammatory genes in chondrocytes, while TA showed no such effect. DEX significantly reduced inflammation but also ECM production in inflamed chondrocytes. At 24 h, DEX treatment led to 168 differentially expressed genes (DEGs) compared to untreated inflamed cells, decreasing to 5 DEGs by 48 h, indicating a rapidly diminishing anti-inflammatory effect. Conversely, the difference between DEX-treated and healthy cells increased over time, from 666 DEGs at 24 h to 1317 DEGs at 48 h. Pathway analysis revealed potential disruptions in cell cycle, mitosis, and ECM homeostasis in DEX-treated cells compared to both healthy and inflamed controls. Interestingly, repeated DEX administration at both a therapeutic (40nM) and a high dose (1µM) induced senescence in healthy cells but not in inflamed cells. In contrast, repeated high-dose DEX reduced apoptosis marker Caspase 3/7 in inflamed but not healthy cells. Despite the transient suppression of inflammation achieved with DEX treatment, the observed decrease in ECM production and induction of senescence in healthy chondrocytes at therapeutic doses, along with apoptosis in inflamed cells at higher doses, underscore the need for caution in its intra-articular administration.
Keywords: Apoptosis; Dexamethasone; Glucocorticoids; Osteoarthritis; Senescence; Triamcinolone.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: No human or animal participants were involved in this study; ovine chondrocytes had been obtained and biobanked from sheep euthanised for reasons unrelated to this study. Based on the “Good Scientific Practice. Ethics in Science und Research” regulation implemented at the University of Veterinary Medicine Vienna, the Institutional Ethics Committee (“Ethics and Animal Welfare Committee”) of the University of Veterinary Medicine Vienna does not require approval of in vitro cell culture studies, if the cells were isolated from tissue, which was obtained either solely for diagnostic or therapeutic purposes or in the course of institutionally and nationally approved experiments. The sheep from which the cells were obtained had been euthanised in the course of a study for which approval of the national (“Commission for Animal Research” of the Austrian Federal Ministry of Science, Research and Economy) and institutional. (“Ethics and Animal Welfare Committee” of the University of Veterinary Medicine Vienna”) animal welfare committees (ethical approval number: 68.205/0100-V/3b/2018) had been granted and which had been reported according to ARRIVE guidelines. All methods were carried out in accordance with the relevant guidelines and regulations.
Figures

References
-
- Bannuru, R. R. et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil.27, 1578–1589 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

