Persistent neutrophilic inflammation is associated with delayed toxicity of phenylarsine oxide in lungs
- PMID: 40195488
- PMCID: PMC11976910
- DOI: 10.1038/s41598-025-95645-z
Persistent neutrophilic inflammation is associated with delayed toxicity of phenylarsine oxide in lungs
Abstract
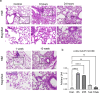
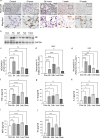
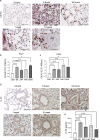
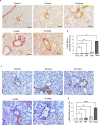
Phenyl arsine oxide (PAO) is a vesicant, similar to Lewisite, a potential chemical warfare agent and an environmental contaminant. PAO-induced skin burns can trigger acute organ injury, including lungs. We have recently demonstrated that PAO burns can have delayed toxicity, although the specific mechanism/s remain to be determined. A single cutaneous exposure to PAO resulted in inflammatory acute lung injury at 6 and 24 h. While acute injury subsiding by 1 week, we observed a significant airway remodeling at 10 weeks post-PAO exposure. The mechanism of prolonged PAO toxicity was associated with the influx of neutrophils that produced harmful neutrophil extracellular traps (NETs). We demonstrated that the crosstalk between NET deployments and expression of IL-33, a pro-remodeling mediator was associated with the development of peribronchial fibrosis. In summary, these results suggest that a single cutaneous exposure to PAO causes the acute inflammatory phase followed by NETs/IL-33 feed forward signaling implicated for the persistent neutrophil influx and NETs formation resulting in airway remodeling.
Keywords: Airway remodeling; Arsenicals; IL-33; Neutrophil extracellular traps; Persistent inflammation; Phenyl Arsine oxide.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval: We obtained human blood neutrophil cells under protocol IRB-300,013,426, which was approved by the Human Subjects Institutional Review Board of the University of Alabama at Birmingham. The University of Alabama at Birmingham Institutional Animal Care and Use Committee approved animal experiments under protocol APN-22866 and performed them in accordance with NIH guidelines.
Figures

Update of
-
Persistent Neutrophilic Inflammation is Associated with Delayed Toxicity of Phenylarsine Oxide in Lungs.Res Sq [Preprint]. 2025 Jan 13:rs.3.rs-5100050. doi: 10.21203/rs.3.rs-5100050/v1. Res Sq. 2025. Update in: Sci Rep. 2025 Apr 7;15(1):11840. doi: 10.1038/s41598-025-95645-z. PMID: 39877099 Free PMC article. Updated. Preprint.
Similar articles
-
Persistent Neutrophilic Inflammation is Associated with Delayed Toxicity of Phenylarsine Oxide in Lungs.Res Sq [Preprint]. 2025 Jan 13:rs.3.rs-5100050. doi: 10.21203/rs.3.rs-5100050/v1. Res Sq. 2025. Update in: Sci Rep. 2025 Apr 7;15(1):11840. doi: 10.1038/s41598-025-95645-z. PMID: 39877099 Free PMC article. Updated. Preprint.
-
A Murine Model of Vesicant-Induced Acute Lung Injury.J Pharmacol Exp Ther. 2024 Jan 17;388(2):568-575. doi: 10.1124/jpet.123.001780. J Pharmacol Exp Ther. 2024. PMID: 38050084 Free PMC article.
-
Cutaneous lewisite exposure causes acute lung injury.Ann N Y Acad Sci. 2020 Nov;1479(1):210-222. doi: 10.1111/nyas.14346. Epub 2020 Apr 24. Ann N Y Acad Sci. 2020. PMID: 32329907 Free PMC article.
-
Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation.Genes (Basel). 2019 Feb 26;10(3):183. doi: 10.3390/genes10030183. Genes (Basel). 2019. PMID: 30813645 Free PMC article. Review.
-
The role of neutrophil extracellular traps in acute lung injury.Front Immunol. 2022 Jul 29;13:953195. doi: 10.3389/fimmu.2022.953195. eCollection 2022. Front Immunol. 2022. PMID: 35967320 Free PMC article. Review.
References
-
- Goldman, L. The vesicant chemical warfare agents. Arch. Derm. Syphilol.42, 123–136 (1940).
-
- Bełdowski, J. et al. Chemical Munitions Search & Assessment—An Evaluation of the Dumped Munitions Problem in the Baltic Sea128 (Deep-Sea Research II, 2016).
-
- Jacek Bełdowski, M. S. et al. Arsenic concentrations in Baltic Sea sediments close to chemical munitions dumpsites. Deep Sea Res. Part II: Top. Stud. Oceanogr.128, 114–122 (2016).
-
- Christensen, I. M. A., Fauser, M. S. S. P., Hansen, S. F., Baatrup, E. & Sanderson, H. Acute toxicity of sea-dumped chemical munitions: luminating the environmental Toxcity of legacy compounds. Global Security: HealtH Sci. Policy1, 39–50 (2016).
-
- Carton, G. J. & Andrzej, J. Historic disposal of munitions in U.S. And European coastal waters, how historic information can be used in characterizing And managing risk. Mar. Technol. Soc. J.43(4), 16–32 (2009).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

