The selenocysteine-containing protein SELENOT maintains dopamine signaling in the midbrain to protect mice from hyperactivity disorder
- PMID: 40195499
- PMCID: PMC12084338
- DOI: 10.1038/s44318-025-00430-3
The selenocysteine-containing protein SELENOT maintains dopamine signaling in the midbrain to protect mice from hyperactivity disorder
Abstract
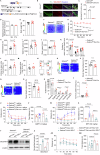
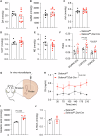
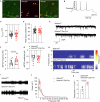
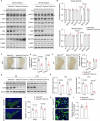
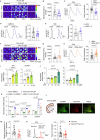
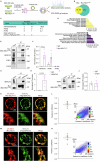
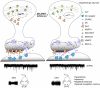
Dopaminergic neuron dysfunction has been implicated in multiple neurological and psychiatric disorders. SELENOT is a selenocysteine-containing protein of the ER membrane with antioxidant and neuroprotective activities, but its pathophysiological role in dopaminergic neurons remains unclear. In this study we show that male mice with SELENOT-deficient dopaminergic neurons exhibit attention deficit/hyperactivity disorder (ADHD)-like symptoms, including hyperlocomotion, recognition memory deficits, repetitive movements, and impulsivity. Dopamine metabolism, extrasynaptic dopamine levels, spontaneous excitatory postsynaptic currents in the striatum, and electroencephalography theta power are all enhanced in these animals, while dopaminergic neurons in the substantia nigra are slightly reduced but with normal firing and cellular stress levels. Our results also indicate that the expression of dopamine transporter (DAT) is significantly reduced in the absence of SELENOT. Both the development of ADHD-like phenotypes and DAT downregulation are also observed when SELENOT is absent from the whole brain, but not when its conditional knockout is restricted to astrocytes. Mechanistically, we show that SELENOT downregulates DAT expression via interaction with SERCA2 of the ER -but not with IP3R or RYR- to regulate the ER-cytosol Ca2+ flux and, subsequently, the activity of transcription factor NURR1 and the expression levels of DAT. Treatment with amphetamine or methylphenidate, which are commonly used to treat ADHD, reverses the hyperactivity observed in mice with SELENOT-deficient dopaminergic neurons. Our study demonstrates that SELENOT in mouse dopaminergic neurons maintains proper dopamine signaling in the midbrain against the development of ADHD-like behaviors.
Keywords: Attention Deficit/Hyperactivity Disorder; Calcium; Dopamine Transporter; Dopaminergic Neurons; SELENOT.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures


References
-
- Boukhzar L, Hamieh A, Cartier D, Tanguy Y, Alsharif I, Castex M, Arabo A, El Hajji S, Bonnet JJ, Errami M et al (2016) Selenoprotein T exerts an essential oxidoreductase activity that protects dopaminergic neurons in mouse models of Parkinson’s disease. Antioxid Redox Signal 24:557–574 - PMC - PubMed
-
- Castex MT, Arabo A, Benard M, Roy V, Le Joncour V, Prevost G, Bonnet JJ, Anouar Y, Falluel-Morel A (2016) Selenoprotein T deficiency leads to neurodevelopmental abnormalities and hyperactive behavior in mice. Mol Neurobiol 53:5818–5832 - PubMed
MeSH terms
Substances
Grants and funding
- 82071585/MOST | National Natural Science Foundation of China (NSFC)
- 82271282/MOST | National Natural Science Foundation of China (NSFC)
- 82471617/MOST | National Natural Science Foundation of China (NSFC)
- ZCLTGD24C0902/MOST | NSFC | NSFC-Zhejiang Joint Fund | | Natural Science Foundation of Zhejiang Province (ZJNSF)
- ZCLZ25H2502/MOST | NSFC | NSFC-Zhejiang Joint Fund | | Natural Science Foundation of Zhejiang Province (ZJNSF)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

