Immunoinformatics-driven design of a multi-epitope vaccine targeting neonatal rotavirus with focus on outer capsid proteins VP4 and VP7 and non structural proteins NSP2 and NSP5
- PMID: 40195509
- PMCID: PMC11976959
- DOI: 10.1038/s41598-025-95256-8
Immunoinformatics-driven design of a multi-epitope vaccine targeting neonatal rotavirus with focus on outer capsid proteins VP4 and VP7 and non structural proteins NSP2 and NSP5
Abstract
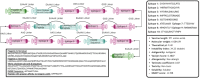
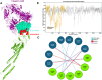
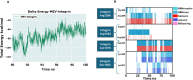
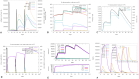
Rotaviral gastroenteritis remains a major global health concern, particularly for infants and young children under five years old. Prior to the introduction of the WHO-prequalified rotavirus vaccine, rotavirus (RV) was responsible for approximately 800,000 child deaths annually in developing countries. Although vaccination efforts have reduced this number, RV still causes around 200,000 child deaths each year worldwide. The current WHO-prequalified vaccines are live attenuated and offer limited efficacy of 40-60%, with a slight risk of intussusception in young children. To overcome these limitations, we employed immunoinformatics to design a novel multi-epitope vaccine (MEV) targeting rotavirus outer capsid proteins VP4 and VP7, as well as crucial non-structural proteins NSP2 and NSP5. The RV-MEV incorporates 10 epitopes, including 4 CD8 + T-cell, 5 CD4 + T-cell, and 1 B-cell epitope, all of which are antigenic, non-allergenic, and non-toxic. These epitopes also showed potential to induce interferon-γ (IFN-γ). Molecular simulation studies confirmed stable interactions between RV-MEV and human TLR5 and integrin αvβ5 complexes. The RV-MEV was successfully cloned into a pET28a(+) vector during in-silico cloning. Immune simulation studies predict a strong immune response to the RV-MEV. Future in vitro and in vivo studies are necessary to validate the vaccine's effectiveness in providing protection against various rotavirus strains in neonates.
Keywords: B-Cell; Capsid protein; Gastroenteritis; Immune simulation; Immunoinformatics; Integrins; Molecular dynamics; Multi-epitope vaccine; Neonates; Non-structural proteins; Rotavirus; T-Cell; Toll-like receptors (TLRs).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures







References
-
- Gupta, P., Harish, R., PREVALENCE AND CLINICAL PROFILE OF ROTAVIRUS ASSOCIATED & DIARRHEA IN A TERTIARY CARE CENTER IN NORTH INDIA. A DESCRIPTIVE HOSPITAL-BASED STUDY. Indian J. Child. Health (Bhopal). 6, 221–224 (2019).
-
- Bernstein, D. I. Rotavirus overview. Pediatr. Infect. Disease J.28, S50–S53 (2009). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

