Emerging T-cell lymphomas after CAR T-cell therapy
- PMID: 40195525
- PMCID: PMC12133562
- DOI: 10.1038/s41375-025-02574-x
Emerging T-cell lymphomas after CAR T-cell therapy
Conflict of interest statement
Competing interests: MH gave advisory boards and received honoraria from Abbvie, Beigene, Jazz, Janssen, Stemline Menarini, and Takeda, and received research support from EDO-Mundipharma, Janpix, Novartis, and Roche. MM: Advisory Boards/Honoraria/Research support: Amgen, BMS, Celgene, Gilead, Janssen, Stemline, Springworks, and Takeda.
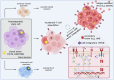
Figures
References
-
- Locke FL, Miklos DB, Jacobson CA, Perales M-A, Kersten M-J, Oluwole OO, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med. 2022;386:640–54. - PubMed
-
- San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos M-V, et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N Engl J Med. 2023;389:335–47. - PubMed
-
- Carvalho T. FDA approves Genentech’s bispecific antibody for lymphoma. Nat Med. 2023;29:507–8. - PubMed
-
- Dulery R, Guiraud V, Choquet S, Thieblemont C, Bachy E, Barete S et al. T cell malignancies after CAR T cell therapy in the DESCAR-T registry. Nat Med. 2025. 10.1038/s41591-024-03458-w. - PubMed
Publication types
Grants and funding
- 70113307/Deutsche Krebshilfe (German Cancer Aid)
- 2023.084.1/Wilhelm Sander-Stiftung (Wilhelm Sander Foundation)
- 08 R/2023/José Carreras Leukämie-Stiftung (Deutsche José Carreras Leukämie-Stiftung)
- 01 R_2023/José Carreras Leukämie-Stiftung (Deutsche José Carreras Leukämie-Stiftung)
- SaxoCell/Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Federal Ministry for Education, Science, Research and Technology)
LinkOut - more resources
Full Text Sources

