This is a preprint.
Targeting senescence in Amyotrophic Lateral Sclerosis: senolytic treatment improves neuromuscular function and preserves cortical excitability in a TDP-43Q331K mouse model
- PMID: 40196013
- PMCID: PMC11975006
- DOI: 10.21203/rs.3.rs-6081213/v1
Targeting senescence in Amyotrophic Lateral Sclerosis: senolytic treatment improves neuromuscular function and preserves cortical excitability in a TDP-43Q331K mouse model
Abstract
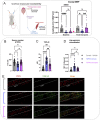
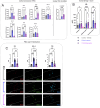
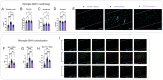
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder marked by progressive motor neuron degeneration in the primary motor cortex (PMC) and spinal cord. Aging is a key factor in ALS onset and progression, with evidence suggesting that biological aging-a process involving cellular decline- far outpaces chronological aging in ALS. This promotes senescent cell accumulation-marked by irreversible cell-cycle arrest, impaired apoptosis, and chronic inflammation-disrupting tissue homeostasis and impairing neuronal support functions. Thus, targeting senescence presents a novel therapeutic strategy for ALS. Here, we investigated the senolytic combination Dasatinib and Quercetin (D&Q) in TDP-43Q331K ALS mice. D&Q improved neuromuscular function and reduced plasma neurofilament light chain, a biomarker of axonal damage. The most pronounced improvement was the improved cortical excitability, accompanied by reductions in senescence and TDP-43 in the PMC. These findings highlight the potential of senolytics to mitigate ALS-related dysfunction, supporting their viability as a therapeutic strategy.
Keywords: ALS; aging; motor cortex; neuromuscular; senescence; senolytics.
Conflict of interest statement
DISCLOSURES The authors have no conflicts of interest to report.
Figures



Similar articles
-
Phagocytosis of aggrecan-positive perineuronal nets surrounding motor neurons by reactive microglia expressing MMP-9 in TDP-43Q331K ALS model mice.Neurobiol Dis. 2024 Oct 1;200:106614. doi: 10.1016/j.nbd.2024.106614. Epub 2024 Jul 25. Neurobiol Dis. 2024. PMID: 39067491
-
Complement components are upregulated and correlate with disease progression in the TDP-43Q331K mouse model of amyotrophic lateral sclerosis.J Neuroinflammation. 2018 Jun 1;15(1):171. doi: 10.1186/s12974-018-1217-2. J Neuroinflammation. 2018. PMID: 29859100 Free PMC article.
-
Defects in synaptic transmission at the neuromuscular junction precede motor deficits in a TDP-43Q331K transgenic mouse model of amyotrophic lateral sclerosis.FASEB J. 2018 May;32(5):2676-2689. doi: 10.1096/fj.201700835R. Epub 2018 Jan 2. FASEB J. 2018. PMID: 29295857
-
Chronological and Biological Aging in Amyotrophic Lateral Sclerosis and the Potential of Senolytic Therapies.Cells. 2024 May 28;13(11):928. doi: 10.3390/cells13110928. Cells. 2024. PMID: 38891059 Free PMC article. Review.
-
Senolytics: A Novel Strategy for Neuroprotection in ALS?Int J Mol Sci. 2021 Nov 8;22(21):12078. doi: 10.3390/ijms222112078. Int J Mol Sci. 2021. PMID: 34769512 Free PMC article. Review.
References
-
- Das MM, Svendsen CN (2015) Astrocytes show reduced support of motor neurons with aging that is accelerated in a rodent model of ALS. Neurobiol Aging 36:1130–1139 - PubMed
-
- Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andrés V (2020) Biological Versus Chronological Aging: JACC Focus Seminar. J Am Coll Cardiol 75:919–930 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

