Cutaneous Adverse Events Following COVID-19 Vaccination in Japan: A Questionnaire Survey
- PMID: 40196074
- PMCID: PMC11975142
- DOI: 10.7759/cureus.80257
Cutaneous Adverse Events Following COVID-19 Vaccination in Japan: A Questionnaire Survey
Abstract
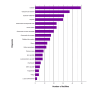
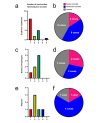
We conducted a nationwide survey in Japan to clarify the clinical spectrum of these events. An initial questionnaire was sent to 126 dermatology facilities, and responses were obtained from 66 (52.4%). Among these responding facilities, the most commonly identified cutaneous adverse events after COVID-19 vaccination were urticaria (49 (74.2%)), delayed local reactions (37 (56.1%)), erythema multiforme (31 (47.0%)), and alopecia (30 (45.5%)). In the detailed survey, the primary adverse events were EM (19 (20.9%)), bullous pemphigoid (7 (7.7%)), and alopecia (6 (6.6%)). The mean latency from vaccination to onset was 13.1 days, and the mean duration of symptoms was 74.2 days. Although this study cannot establish a direct causal relationship between vaccination and adverse events, it highlights the need for dermatologists to recognize potential cutaneous reactions and provide appropriate care.
Keywords: alopecia; bullous pemphigoid; covid-19 vaccine; cutaneous adverse events; erythema multiforme; psoriasis.
Copyright © 2025, Yamamura et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. The Ethics Committee of Aichi Medical University Hospital issued approval 2022-233. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: This research was funded by MHLW Research on Emerging and Re-emerging Infectious Diseases and Immunization, under Program Grant Number JPMH21HA2011 and JPMH23HA2011. Financial relationships: Norito Katoh declare(s) personal fees from Pfizer. Norito Katoh has received honoraria as a speaker/consultant for Pfizer. . Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Corona virus: a review of Covid-19. Kumar D, Malviya R, Sharma PK. https://accscience.com/journal/EJMO/4/1/10.14744/ejmo.2020.51418 Eurasian J Med Oncol. 2020;4:8–25.
-
- Developing a vaccine for COVID-19. Caddy S. BMJ. 2020;369:0. - PubMed
-
- Effectiveness of mRNA COVID-19 vaccines in Japan during the nationwide pandemic of the Delta variant. Akaishi T, Kushimoto S, Katori Y, et al. Tohoku J Exp Med. 2022;257:1–6. - PubMed
LinkOut - more resources
Full Text Sources
