Mutations in ace2 gene modulate cytokine levels and alter immune responses in Mycobacterium tuberculosis and SARS-CoV-2 co-infection: a Cameroonian cohort
- PMID: 40196114
- PMCID: PMC11973369
- DOI: 10.3389/fimmu.2025.1533213
Mutations in ace2 gene modulate cytokine levels and alter immune responses in Mycobacterium tuberculosis and SARS-CoV-2 co-infection: a Cameroonian cohort
Abstract
Introduction: SARS-CoV-2 and Mycobacterium tuberculosis (Mtb) share similarities in their modes of transmission, pathophysiological symptoms, and clinical manifestations. An imbalance in the immune response characterised by elevated levels of some inflammatory cytokines caused by tuberculosis (TB) and COVID-19 may increase the risk of developing a severe disease-like condition. It has been reported that TB increases the expression levels of Ace2 (angiotensin converting enzyme 2) and Tmprss2 (transmembrane protease serine 2) proteins, which are essential for COVID-19 pathogenesis. Single nucleotide polymorphisms (SNPs) variants of ace2 and tmprss2 genes can impact virus and host-cell interactions and alter immune responses by modulating cytokine production. This may modify the susceptibility and/or severity in COVID-19-infected people. The role of SNPs in ace2 and tmprss2 in relation to Mtb and SARS-CoV-2 co-infection is relatively underexplored.
Method: In this study, genotype frequency of 10 SNPs of ace2 and 03 SNPs of tmprss2 genes in a Cameroonian cohort consisting of COVID-19-positive (n = 31), TB-positive (n = 43), TB-COVID-19 co-infected (n = 21), and a control group (n = 24) were studied. The immune response was estimated by quantitating inflammatory cytokine levels alongside self-reported and clinically diagnosed symptoms. The relationship between specific genetic mutations in these ace2 gene SNPs and their impact on cytokine expression levels in Mtb and SARS-CoV-2 co-infected patients was investigated.
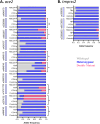
Results: We identified wild-type, heterozygous, and double-mutant genotypes in seven SNPs (rs2285666, rs6632677, rs4646116, rs4646140, rs147311723, rs2074192 and rs4646142) in ace2 gene, which showed significant variations in distribution across the study groups. Our most significant findings include the association of double mutant alleles (AA) of rs4646140 and rs2074192 in the ace2 gene with decreased IL-6 and IL-2 expression levels respectively in TB-COVID-19 participants. Also, the double mutant alleles (AA) of rs4646116 were responsible for increased expression level of IL-2 in TB-COVID-19 patients. Additionally, elevated serum levels of AST, urea, and D-dimer, as well as increased plasma concentrations of IL-10, IFN-γ, and TNF-α, have been associated with co-infections involving Mtb and SARS-CoV-2.
Conclusion: These biomarkers may reflect the complex interplay between the two pathogens and their impact on host immune responses and disease progression. This study highlights the critical role of genetic and immunological factors in shaping altered immune responses during co-infections involving Mtb and SARS-CoV-2. By elucidating these factors, the findings provide a foundation for a deeper understanding of host-pathogen interactions and their implications for disease progression and outcomes. Furthermore, this research has the potential to drive advancements in diagnostic approaches enabling more accurate detection and monitoring of co-infections.
Keywords: COVID - 19; Mycobacterium tuberculosis; SARS-CoV-2; SNPs; ace2; immune response; tmprss2; tuberculosis.
Copyright © 2025 Kameni, Tchoupe, Kamdem, Bhalla, Assam Assam, Tepa, Neba, Nanda, Awuah, Amuasi and Netongo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Genetic Association between ACE2 (rs2285666 and rs2074192) and TMPRSS2 (rs12329760 and rs2070788) Polymorphisms with Post-COVID Symptoms in Previously Hospitalized COVID-19 Survivors.Genes (Basel). 2022 Oct 24;13(11):1935. doi: 10.3390/genes13111935. Genes (Basel). 2022. PMID: 36360172 Free PMC article.
-
Polymorphisms and mutations of ACE2 and TMPRSS2 genes are associated with COVID-19: a systematic review.Eur J Med Res. 2022 Feb 22;27(1):26. doi: 10.1186/s40001-022-00647-6. Eur J Med Res. 2022. PMID: 35193695 Free PMC article.
-
ACE2 and TMPRSS2 SNPs as Determinants of Susceptibility to, and Severity of, a COVID-19 Infection.Br J Biomed Sci. 2022 Jan 12;79:10238. doi: 10.3389/bjbs.2021.10238. eCollection 2022. Br J Biomed Sci. 2022. PMID: 35996506 Free PMC article.
-
Role of ACE2 and TMPRSS2 polymorphisms on COVID-19 outcome and disease severity in adult patients: A prospective cohort study in a tertiary hospital, Indonesia.Narra J. 2024 Aug;4(2):e919. doi: 10.52225/narra.v4i2.919. Epub 2024 Aug 12. Narra J. 2024. PMID: 39280326 Free PMC article.
-
Contributions of human ACE2 and TMPRSS2 in determining host-pathogen interaction of COVID-19.J Genet. 2021;100(1):12. doi: 10.1007/s12041-021-01262-w. J Genet. 2021. PMID: 33707363 Free PMC article. Review.
References
-
- Global Tuberculosis Report 2023. 1st ed. Geneva: World Health Organization; (2023).
-
- Lampart M, Zellweger N, Bassetti S, Tschudin-Sutter S, Rentsch KM, Siegemund M, et al. . Clinical utility of inflammatory biomarkers in COVID-19 in direct comparison to other respiratory infections—A prospective cohort study. PloS One. (2022) 17:e0269005. doi: 10.1371/journal.pone.0269005 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

