Telitacicept as an alternative to non-steroidal immunosuppressive therapies in the treatment of myasthenia gravis: a study on clinical efficacy and steroid-sparing effect
- PMID: 40196119
- PMCID: PMC11973063
- DOI: 10.3389/fimmu.2025.1549034
Telitacicept as an alternative to non-steroidal immunosuppressive therapies in the treatment of myasthenia gravis: a study on clinical efficacy and steroid-sparing effect
Abstract
Introduction: Myasthenia Gravis (MG) is an autoimmune disorder characterized by impaired neuromuscular junction (NMJ) transmission. Current treatments for MG include steroids and nonsteroidal immunosuppressive therapies (NSISTs). However, approximately 20% of patients show a poor response to these therapies, which are often associated with significant side effects. Telitacicept, a novel recombinant fusion protein targeting the BAFF/APRIL pathway, has shown promise in treating autoimmune diseases, including MG.
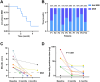
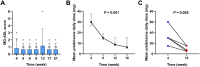
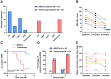
Methods: This retrospective study compared the efficacy of telitacicept monotherapy (10 patients) to NSISTs (16 patients) and sequential therapy (6 patients) in managing Myasthenia Gravis (MG) at The First Affiliated Hospital of Wenzhou Medical University (July 2020-November 2024). The primary endpoint was the time to achieve minimal symptom expression (MSE), and secondary endpoint was the change in the mean daily prednisone dosage from baseline to month 4.
Results: Among telitacicept-treated patients, 80% achieved MSE within 4 months, with a significant reduction in mean daily dose of prednisone (from 45.00 mg to 6.25 mg, P < 0.001). In contrast, only 12.5% of the NSISTs group achieved MSE, with no significant change in mean daily dose of prednisone (P = 0.091). The sequential therapy group (efgartigimod followed by telitacicept) maintained stable disease conditions.
Conclusion: Telitacicept is effective in inducing MSE rapidly and offers a steroid-sparing effect, making it a promising alternative to traditional NSISTs with fewer side effects in MG patients.
Keywords: minimal symptom expression; myasthenia gravis; non-steroidal immunosuppressive therapies; steroid-sparing; telitacicept.
Copyright © 2025 Fang, Zhang, Zhang, Zhang, Qu, Pan, Wan, Yang, Zhang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

