This is a preprint.
Whole-Genome Sequencing Reveals Individual and Cohort Level Insights into Chromosome 9p Syndromes
- PMID: 40196253
- PMCID: PMC11974940
- DOI: 10.1101/2025.03.28.25324850
Whole-Genome Sequencing Reveals Individual and Cohort Level Insights into Chromosome 9p Syndromes
Update in
-
Whole-genome sequencing reveals individual and cohort level insights into chromosome 9p syndromes.Genome Med. 2025 Oct 24;17(1):129. doi: 10.1186/s13073-025-01563-0. Genome Med. 2025. PMID: 41137173 Free PMC article.
Abstract
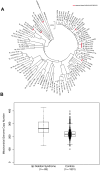
Previous genomic efforts on chromosome 9p deletion and duplication syndromes have utilized low resolution strategies (i.e., karyotypes, chromosome microarrays). We present the first large-scale whole-genome sequencing (WGS) study of 100 individuals from families with 9p-related syndromes including 85 unrelated probands through the 9P-ARCH (Advanced Research in Chromosomal Health: Genomic, Phenotypic, and Functional Aspects of 9p-Related syndromes) research network. We analyzed the genomic architecture of these syndromes, highlighting fundamental features and their commonalities and differences across individuals. This work includes a machine-learning model that predicts 9p deletion syndrome from gene copy number estimates using WGS data. Two Late Replicating Regions (LRR1 [a previously un-named human fragile site], LRR2) were identified that contain most structural variant breakpoints in 9p deletion syndrome pointing to replication-based issues in structural variant formation. Furthermore, we show the utility of using WGS information to obtain a comprehensive understanding of 9p-related variation in an individual with complex structural variation where chromothripsis is the likely mechanism. Genes on 9p were prioritized based on statistical assessment of human genomic variation. Furthermore, through application of spatial transcriptomics to embryonic mouse tissue we examined 9p-gene expression in craniofacial and brain development. Through these strategies, we identified 24 important genes for the majority (83%) of individuals with 9p deletion syndrome including AK3, BRD10, CD274, CDC37L1, DMRT1, DMRT2, DMRT3, DOCK8, GLIS3, JAK2, KANK1, KDM4C, PLPP6, PTPRD, PUM3, RANBP6, RCL1, RFX3, RIC1, SLC1A1, SMARCA2, UHRF2, VLDLR, and ZNG1A. Two genes (AK3, ZNG1A) are involved in mitochondrial function and testing of the mitochondrial genome revealed excess copy number in individuals with 9p deletion syndrome. This study presents the most comprehensive genomic analysis of 9p-related syndromes to date, with plans for further expansion through our 9P-ARCH research network.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
