This is a preprint.
Androgen Deprivation-Induced TET2 Activation Fuels Prostate Cancer Progression via Epigenetic Priming and Slow-Cycling Cancer Cells
- PMID: 40196510
- PMCID: PMC11974783
- DOI: 10.1101/2025.03.26.645495
Androgen Deprivation-Induced TET2 Activation Fuels Prostate Cancer Progression via Epigenetic Priming and Slow-Cycling Cancer Cells
Abstract
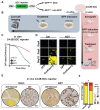
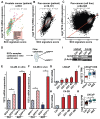
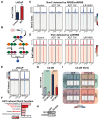
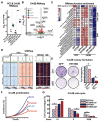
Advanced prostate cancer (PCa) frequently develops resistance to androgen deprivation therapy through various mechanisms including lineage plasticity. Slow-cycling cells (SCCs) have emerged as key players in adaptive responses to therapy, yet their role in PCa remains unclear. Through in silico analysis of single-cell RNA sequencing (scRNA-seq) data, we discovered that SCCs are enriched during pivotal stages of PCa progression, including the transition from androgen-dependent to castration-resistant states and the emergence of neuroendocrine PCa (NEPC). Using a tetracycline-inducible H2BeGFP reporter system, we confirmed SCC enrichment following androgen deprivation in both in vitro and in vivo models. Furthermore, we identified TET2 as a key regulator of SCCs, with its expression upregulated by androgen deprivation and positively correlated with SCC signature scores in PCa. Genome-wide 5-hydroxymethylcytosine (5hmC) profiling revealed increased hydroxymethylation after androgen deprivation, while TET2 knockdown reduced 5hmC levels at specific loci. Functional studies demonstrated that TET2 governs SCC maintenance, cell cycle progression, and DNA damage repair. Targeting TET2, either alone or in combination with an ATM inhibitor, significantly suppressed tumor growth, highlighting TET2 as a promising therapeutic target. Our study provides the first single-nucleotide resolution map of 5hmC dynamics in PCa, identifies a cell state driving epigenetic rewiring, and underscores the transformative potential of novel therapeutic strategies for advanced PCa.
Figures

Similar articles
-
Integrated single-cell transcriptomic analyses identify a novel lineage plasticity-related cancer cell type involved in prostate cancer progression.EBioMedicine. 2024 Nov;109:105398. doi: 10.1016/j.ebiom.2024.105398. Epub 2024 Oct 16. EBioMedicine. 2024. PMID: 39418984 Free PMC article.
-
TET2-mediated 5-hydroxymethylcytosine of TXNIP promotes cell cycle arrest in systemic anaplastic large cell lymphoma.Clin Epigenetics. 2025 Jan 21;17(1):10. doi: 10.1186/s13148-025-01816-0. Clin Epigenetics. 2025. PMID: 39838392 Free PMC article.
-
The long noncoding RNA H19 regulates tumor plasticity in neuroendocrine prostate cancer.Nat Commun. 2021 Dec 21;12(1):7349. doi: 10.1038/s41467-021-26901-9. Nat Commun. 2021. PMID: 34934057 Free PMC article.
-
Lineage plasticity and treatment resistance in prostate cancer: the intersection of genetics, epigenetics, and evolution.Front Endocrinol (Lausanne). 2023 Jun 30;14:1191311. doi: 10.3389/fendo.2023.1191311. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37455903 Free PMC article. Review.
-
Focus on the tumor microenvironment: A seedbed for neuroendocrine prostate cancer.Front Cell Dev Biol. 2022 Jul 22;10:955669. doi: 10.3389/fcell.2022.955669. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35938167 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
