This is a preprint.
Distinct Roles of Somatostatin and Parvalbumin Interneurons in Regulating Predictive Actions and Emotional Responses During Trace Eyeblink Conditioning
- PMID: 40196611
- PMCID: PMC11974708
- DOI: 10.1101/2025.03.23.644831
Distinct Roles of Somatostatin and Parvalbumin Interneurons in Regulating Predictive Actions and Emotional Responses During Trace Eyeblink Conditioning
Abstract
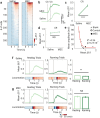
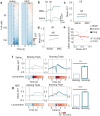
Learning involves evaluating multiple dimensions of information and generating appropriate actions, yet how the brain assigns value to this information remains unclear. In this study, we show that two types of interneurons (INs) in the primary somatosensory cortex-somatostatin-expressing (SST-INs) and parvalbumin-expressing (PV-INs) neurons-differentially contribute to information evaluation during trace eyeblink conditioning (TEC). An air puff (unconditioned stimulus, US) delivered after a whisker stimulus (conditioned stimulus, CS) elicited both reflexive eye closure and stress-related locomotion. However, only self-initiated, anticipatory eye closure during the CS window, measured via electromyography (EMG), was directly relevant to learning performance. We found that SST-IN activity changes aligned with the learning induced changes of the anticipatory eye blinks during the CS period, correlated with the EMG changes across learning. In contrast, PV-IN activity was positively correlated with stress-related locomotion following the US and showed no learning related changes, suggesting a role in processing the emotional or aversive component of the task. Furthermore, cholinergic signaling via nicotinic receptors modulated both SST- and PV-IN activities, in a manner consistent with their distinctive roles, linking these interneurons to the regulation of learning-related actions and emotional responses, respectively. These findings demonstrate that distinct interneuron populations evaluate different dimensions of information-SST-INs for predictive, adaptive actions and PV-INs for stress-related emotional responses-to guide learning and behavior.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Similar articles
-
Learning induced neuronal identity switch in the superficial layers of the primary somatosensory cortex.bioRxiv [Preprint]. 2023 Sep 1:2023.08.30.555603. doi: 10.1101/2023.08.30.555603. bioRxiv. 2023. PMID: 37693620 Free PMC article. Preprint.
-
Sustained Activity of Hippocampal Parvalbumin-Expressing Interneurons Supports Trace Eyeblink Conditioning in Mice.J Neurosci. 2022 Nov 2;42(44):8343-8360. doi: 10.1523/JNEUROSCI.0834-22.2022. Epub 2022 Sep 27. J Neurosci. 2022. PMID: 36167784 Free PMC article.
-
Conditional learning increases inhibition of layer 4 excitatory neurons by somatostatin- and parvalbumin-expressing interneurons in the barrel cortex.J Neural Transm (Vienna). 2025 May 30. doi: 10.1007/s00702-025-02949-5. Online ahead of print. J Neural Transm (Vienna). 2025. PMID: 40445322
-
Prefrontal GABAergic Interneurons Gate Long-Range Afferents to Regulate Prefrontal Cortex-Associated Complex Behaviors.Front Neural Circuits. 2021 Jul 12;15:716408. doi: 10.3389/fncir.2021.716408. eCollection 2021. Front Neural Circuits. 2021. PMID: 34322002 Free PMC article. Review.
-
Cortical interneurons: fit for function and fit to function? Evidence from development and evolution.Front Neural Circuits. 2023 May 4;17:1172464. doi: 10.3389/fncir.2023.1172464. eCollection 2023. Front Neural Circuits. 2023. PMID: 37215503 Free PMC article. Review.
References
-
- Christian K. M. and Thompson R. F. (2005). Long-term storage of an associative memory trace in the cerebellum. Behav Neurosci, 119, 526–537. - PubMed
-
- Del Rosario J., Coletta S., Kim S. H., Mobille Z., Peelman K., Williams B., Otsuki A. J., Del Castillo Valerio A., Worden K., Blanpain L. T., Lovell L., Choi H. and Haider B. (2025). Lateral inhibition in V1 controls neural and perceptual contrast sensitivity. Nat Neurosci. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
