This is a preprint.
Placental Igf1 Overexpression Sex-Specifically Impacts Mouse Placenta Structure, Altering Offspring Striatal Development and Behavior
- PMID: 40196637
- PMCID: PMC11974794
- DOI: 10.1101/2025.03.27.644829
Placental Igf1 Overexpression Sex-Specifically Impacts Mouse Placenta Structure, Altering Offspring Striatal Development and Behavior
Update in
-
Placental Igf1 overexpression sex-specifically impacts mouse placenta structure, altering offspring striatal development and behavior.Exp Neurol. 2025 Dec;394:115453. doi: 10.1016/j.expneurol.2025.115453. Epub 2025 Sep 2. Exp Neurol. 2025. PMID: 40907731
Abstract
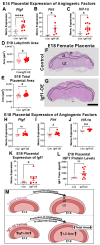
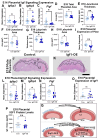
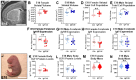
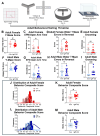
Insulin-like growth factor 1 (IGF1) is produced primarily in the placenta in utero and is an essential hormone for neurodevelopment. Specifically, how placental IGF1 production persistently influences the brain is unclear. This study evaluated the effects of placental Igf1 overexpression on embryonic and postnatal brain development, particularly for striatum, a region highly linked to neurodevelopmental disorders. Placental Igf1 was overexpressed via placental-targeted CRISPR manipulation. This overexpression altered placenta structure and function distinctly in females and males. Early differences in placental function altered the trajectory of striatal development, as adult females showed persistent changes in striatal cell composition and striatal dependent behavior while males were less affected in brain and behavior outcomes. Overall, these results demonstrate that placental Igf1 expression alters striatal development and behavior in ways relevant to neurodevelopmental disorders. These findings expand our understanding of placental influence on neurodevelopment and will aid in identifying placental-targeted preventive interventions.
Keywords: CRISPR; Ganglionic Eminence; Insulin-like Growth Factor 1; Neurodevelopment; Neurodevelopmental Disorders; Neuroplacentology; Placenta; Striatum.
Conflict of interest statement
DECLARATION OF INTERESTS The authors have no interests to declare.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
