This is a preprint.
Optoretinography reveals rapid rod photoreceptor movement upon photoisomerization
- PMID: 40196674
- PMCID: PMC11974685
- DOI: 10.1101/2025.03.22.644466
Optoretinography reveals rapid rod photoreceptor movement upon photoisomerization
Abstract
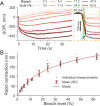
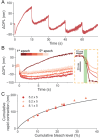
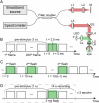
Rod photoreceptors are essential for vision under dim light conditions. The onset of rod-mediated vision is marked by the isomerization of rhodopsin. Here we demonstrate that human and rodent rods undergo a minute and rapid contraction of their outer segments immediately upon photoisomerization. The contraction is explained as an electro-mechanical manifestation of the rod early receptor potential generated in the disk membranes, which is challenging to access in electrophysiology. The bleach-strength dependence of the contraction was accounted by a voltage-dependent membrane tension model, developed earlier to explain a similar behavior in cones. The in vivo optical imaging of light-evoked electrical activity in rodent rods was facilitated by an ultrahigh-resolution point-scan optical coherence tomography (OCT) system coupled with unsupervised learning, while in humans, an adaptive optics line-scan OCT facilitated high-speed recordings in individual rods. The non-invasive in vivo optical imaging of rhodopsin activation will have a significant impact on diagnostics and treatment of retinal disease, especially given the vulnerability of rods in inherited and age-related macular degeneration.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
