Post-viral lung diseases: the microbiota as a key player
- PMID: 40196711
- PMCID: PMC11973713
- DOI: 10.1183/23120541.00560-2024
Post-viral lung diseases: the microbiota as a key player
Abstract
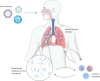
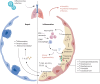
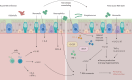
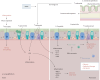
Viral infections of the respiratory tract can lead to chronic lung injury through immunopathological mechanisms that remain unclear. Communities of commensal bacteria colonising the respiratory tract, known as the respiratory tract microbiota, are altered in viral infections, which can contribute to inflammation, lung epithelial damage and subsequent development of lung disease. Emerging evidence on post-viral lung injury suggests an interplay between viral infections, immune responses and airway microbiota composition in the development of viral-induced lung diseases. In this review, we present the clinical characteristics of post-viral lung injury, along with the underlying immunopathological mechanisms and host-bacteria interactions, with a focus on influenza virus, respiratory syncytial virus and coronaviruses. Additionally, considering the important role of the airway microbiota in viral-induced pulmonary sequelae, we suggest key areas for future research on respiratory microbiota involvement in the development of post-viral lung diseases.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: S.V. Stadler reports grants from MD Boursary (Faculty of Biology and Medicine, University of Lausanne, Lausanne, Switzerland and Fondation CHUV, Lausanne University Hospital, Lausanne, Switzerland), disclosures made outside the submitted work. Conflict of interest: C. von Garnier reports grants from OM Pharma; consultancy fees from AstraZeneca, GSK, Boehringer Ingelheim and OM Pharma; payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GSK and OM Pharma, all disclosures made outside the submitted work. Conflict of interest: N.D. Ubags reports grants from OM Pharma; and is European Respiratory Society Assembly 3 Secretary of Group 3.03 and former Chair of the ERS Early Career Member Committee, all disclosures made outside the submitted work.
Figures
Similar articles
-
Respiratory Disease following Viral Lung Infection Alters the Murine Gut Microbiota.Front Immunol. 2018 Feb 12;9:182. doi: 10.3389/fimmu.2018.00182. eCollection 2018. Front Immunol. 2018. PMID: 29483910 Free PMC article.
-
Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence.mBio. 2020 Feb 18;11(1):e03236-19. doi: 10.1128/mBio.03236-19. mBio. 2020. PMID: 32071269 Free PMC article.
-
Altered Respiratory Microbiomes, Plasma Metabolites, and Immune Responses in Influenza A Virus and Methicillin-Resistant Staphylococcus aureus Coinfection.Microbiol Spectr. 2023 Aug 17;11(4):e0524722. doi: 10.1128/spectrum.05247-22. Epub 2023 Jun 15. Microbiol Spectr. 2023. PMID: 37318361 Free PMC article.
-
The triad: respiratory microbiome - virus - immune response in the pathophysiology of pulmonary viral infections.Expert Rev Respir Med. 2021 May;15(5):635-648. doi: 10.1080/17476348.2021.1893168. Epub 2021 Mar 1. Expert Rev Respir Med. 2021. PMID: 33605840 Review.
-
Lower Airway Virology in Health and Disease-From Invaders to Symbionts.Medicina (Kaunas). 2018 Oct 13;54(5):72. doi: 10.3390/medicina54050072. Medicina (Kaunas). 2018. PMID: 30344303 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
