Delaying disease progression in COPD with early escalation to triple therapy: a modelling study (DEPICT-2)
- PMID: 40196713
- PMCID: PMC11973711
- DOI: 10.1183/23120541.00438-2024
Delaying disease progression in COPD with early escalation to triple therapy: a modelling study (DEPICT-2)
Abstract
Introduction: In patients with COPD, dual bronchodilator (long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA)) and triple therapy (inhaled corticosteroid/LAMA/LABA) reduce the risk of exacerbations and lung function decline in the short-mid-term, but their long-term impact is unknown. This modelling study explores long-term impact of these therapies on lung function decline, quality of life (QoL) and all-cause mortality.
Methods: This modelling approach used a longitudinal nonparametric superposition model using published data regarding exacerbations, QoL (assessed by St George's Respiratory Questionnaire (SGRQ)) and mortality. The model simulated disease progression from 40 to 75 years of age and assessed the impact of initiating dual bronchodilator at age 45 years ("LAMA/LABA only" group) and escalation to triple therapy at age 50 years ("Escalation to triple" group) on forced expiratory volume in 1 s (FEV1) decline, QoL and mortality.
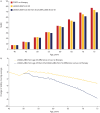
Results: Model simulation predicted that by 75 years of age, "LAMA/LABA only" preserves 159.1 mL of FEV1 versus no treatment, while "Escalation to triple" preserves an additional 376.5 mL and 217.3 mL of FEV1 versus no pharmacotherapy and "LAMA/LABA only", respectively. In "LAMA/LABA only", the SGRQ score reduces (-3.2) versus no treatment, which further reduces to -7.5 in "Escalation to triple". In "LAMA/LABA only", mortality reduces by 5.4% by 75 years versus no treatment, while the "Escalation to triple" shows further decrease in mortality by 12.0%.
Conclusion: Early pharmacotherapy initiation and escalation from dual bronchodilator to triple therapy could slow disease progression by preserving lung function and improving QoL and survival in patients with COPD.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: D. Singh has received consultancy fees from Aerogen, AstraZeneca, BIAL, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, EpiEndo, Genentech, GlaxoSmithKline, Glenmark, Gossamer Bio, Kinaset Therapeutics, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Teva, Theravance Biopharma and Verona Pharma. Conflict of interest: D.F. Litewka has received speaker fees from GSK, Novartis, AstraZeneca, Sanofi, Boehringer Ingelheim and Tuteur and has participated in advisory boards by GSK. Conflict of interest: J.B. Soriano and H. Günen declare no conflicts of interest in this work. Conflict of interest: A. Rendon has received consulting and speaker fees from, and participated in advisory boards for GSK, Boehringer Ingelheim, AstraZeneca, Chiesi and Sanofi; and has received travel support from Chiesi and GSK. Conflict of interest: F.L. Arrabal Fernandes has received consulting fees and travel support from GSK, Boehringer Ingelheim and AstraZeneca and speaker fees from Abbott, GSK, AstraZeneca, Boehringer Ingelheim, Sanofi and Chiesi; has participated in advisory boards by GSK, Boehringer Ingelheim and Sanofi; and is a part of Brazilian Respiratory Society. Conflict of interest: R. Páramo-Arroyo has received consulting fees, grants/contracts and travel support from GSK; speaker fees from GSK, AstraZeneca and Silanes; holds royalties/licenses from GSK, AstraZeneca and Lopmont; and was a part of Sociedad Mexicana de Neumología. Conflict of interest: T. Trinidad has received consulting fees from Orient Euro Pharmaceutical Philippines; speaker fees and travel support from GSK, Orient Euro Pharmaceutical Philippines and United American Philippines; and served on the advisory of Department of Health Philippines (Clinical Practice Guideline for COPD). Conflict of interest: S. Acharya, B. Aggarwal, G. Levy, C. Compton and A. El Hasnaoui are employees of GSK and hold GSK shares. Conflict of interest: P. Daley-Yates received consulting fees from GSK for data analysis related to this modelling study.
Figures


References
LinkOut - more resources
Full Text Sources
