Comparative Clinical and Imaging-Based Evaluation of Therapeutic Modalities in CNS Embryonal Tumours With PLAGL Amplification
- PMID: 40196918
- PMCID: PMC11976507
- DOI: 10.1111/nan.70015
Comparative Clinical and Imaging-Based Evaluation of Therapeutic Modalities in CNS Embryonal Tumours With PLAGL Amplification
Abstract
Aims: Embryonal tumours with PLAGL1 or PLAGL2 amplification (ET, PLAGL) show substantial heterogeneity regarding their clinical characteristics and have been treated inconsistently, resulting in diverse outcomes. In this study, we aimed to evaluate the clinical behaviour of ET, PLAGL and elucidate their response pattern across the different applied treatment regimens.
Methods: We conducted an in-depth retrospective analysis of clinical and serial imaging data of 18 patients with ET, PLAGL (nine each of PLAGL1 and PLAGL2 amplified).
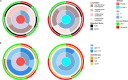
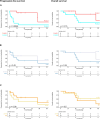
Results: Patients with PLAGL1-amplified tumours (ET, PLAGL1) had fewer relapses (3/9), while PLAGL2-amplified tumours (ET, PLAGL2) were prone to early relapse or progression (8/9) and to distant, leptomeningeal and intraventricular relapses. Progression-free survival differed significantly between the subtypes (log-rank test, p = 0.0055). Postoperative treatment included chemotherapy (n = 17, various protocols), alone (n = 8) or combined with radiotherapy (n = 9). Responses to chemotherapy were observed in both subtypes, and incomplete resection was not associated with inferior survival. All three survivors with ET, PLAGL2 were treated with induction and high-dose chemotherapy with (n = 1-low-dose CSI and boost) or without (n = 2) radiotherapy, whereas five patients with less intensive chemotherapy relapsed. All six survivors with ET, PLAGL1 were treated with conventional chemotherapy regimens, with (n = 4-local radiotherapy n = 3; CSI and boost n = 1) or without (n = 2) radiotherapy. Two patients with ET, PLAGL1 relapsed after 8 years.
Conclusions: Adjuvant therapy should be considered for all ET, PLAGL patients: Patients with ET, PLAGL2 might benefit from intensified chemotherapy regimens. In contrast, patients with ET, PLAGL1 showed superior outcomes without high-dose chemotherapy or craniospinal irradiation.
Keywords: PLAGL1; PLAGL2; ET, PLAGL; embryonal CNS tumour; treatment.
© 2025 The Author(s). Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of British Neuropathological Society.
Conflict of interest statement
The corresponding authors report no potential conflict of interest. Per Nyman reports minority private shareholding (SyntheticMR AB). Laura S. Korhonen is a NOPHO (Nordic Society of Paediatric Haematology) board member. Pieter Wesseling received a travel grant from Chimerix for contributing to a session during the Annual SNO 2023 meeting in Vancouver and is chair of the cIMPACT‐NOW Steering Committee. Ingrid Øra is supported by the Swedish Childhood Cancer Fund (Stockholm, Sweden). Christof M. Kramm is supported by the Deutsche Kinderkrebsstiftung (Bonn, Germany) and has received grants/contracts from Blueprint Rover and Novartis. He is also on the advisory board on glioblastoma medication (Boehringer Ingelheim), chairman of the HIT‐HGG study group, (Goettingen, Germany), executive committee member of the SIOPE DIPG Registry, Utrecht, the Netherlands, and executive committee member of the Neurooncological Working Group (Berlin, Germany). Juhana Hakumäki is president of the Finnish Radiological Society. Michal Zapotocky has received an honorarium from AstraZeneca. Where authors are identified as personnel of the IARC/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policies or views of the IARC/WHO.
Figures

References
-
- Pfister S. M., Reyes‐Mugica M., Chan J. K. C., et al., “A Summary of the Inaugural WHO Classification of Pediatric Tumors: Transitioning From the Optical Into the Molecular Era,” Cancer Discovery 12, no. 2 (2022): 331–355, 10.1158/2159-8290.CD-21-1094. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

