Methods to evaluate the performance of a multicomponent meningococcal serogroup B vaccine
- PMID: 40197090
- PMCID: PMC12039234
- DOI: 10.1128/msphere.00898-24
Methods to evaluate the performance of a multicomponent meningococcal serogroup B vaccine
Abstract
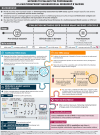
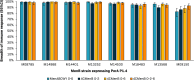
Meningococcal serogroup B (MenB) vaccine licensure was based on the assessment of vaccine-induced immune responses by human serum bactericidal antibody (hSBA) assay against a small number of antigen-specific strains complemented by strain coverage predictions. However, the evaluation of vaccine strain coverage is challenging because of genotypic and phenotypic diversity in surface-exposed MenB strain antigens. This narrative review considers the principal methods applied to assess the performance of a multicomponent MenB vaccine at different stages of its development. Traditional hSBA assay against a limited panel of strains is useful at all stages, while predicted strain coverage methods, such as the meningococcal antigen typing system, are used independent of clinical trials. A new method, the endogenous complement hSBA assay, has been developed to evaluate a vaccine's ability to induce a bactericidal immune response in clinical trials, in conditions that approximate real-world settings through the use of each vaccinee's serum as a source of complement and by testing against a panel of 110 epidemiologically representative MenB strains. Each assay, therefore, has a different scope during the vaccine's development and all complement each other, enabling comprehensive evaluation of the performance of multicomponent MenB vaccines, in advance of real-world evidence of vaccine effectiveness and vaccine impact.
Keywords: 4CMenB; Neisseria meningitidis; invasive meningococcal disease; serum bactericidal antibody assay; vaccine; vaccine effectiveness.
Conflict of interest statement
R.B. reports contract research on behalf of UKHSA for GSK, Pfizer, and Sanofi. L.T.C., D.T., S. Bambini, S. Bobde, A.B., and M.L. are employed by GSK and hold financial equities in GSK. W.-Y.S. and V.M. were employed by GSK at the time of manuscript development. P.T.B. is an inventor on patents related to meningococcal serogroup B vaccines. The authors declare no other financial and non-financial relationships and activities.
Figures

References
-
- US Food and Drug Administration . 2019. Demonstrating substantial evidence of effectiveness for human drug and biological products. Available from: www.fda.gov/regulatory-information/search-fda-guidance-documents/demonst...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

