An injectable multifunctional nanocomposite hydrogel promotes vascularized bone regeneration by regulating macrophages
- PMID: 40197239
- PMCID: PMC11978117
- DOI: 10.1186/s12951-025-03358-2
An injectable multifunctional nanocomposite hydrogel promotes vascularized bone regeneration by regulating macrophages
Abstract
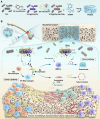
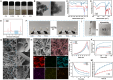
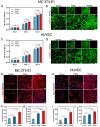
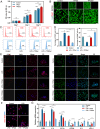
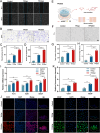
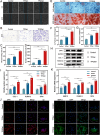
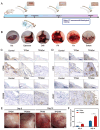
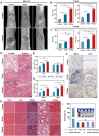
The local inflammatory microenvironment, insufficient vascularization, and inadequate bone repair materials are the three key factors that constrain the repair of bone defects. Here, we synthesized a composite nanoparticle, TPQ (TCP-PDA-QK), with a core‒shell structure. The core consists of nanotricalcium phosphate (TCP), and the shell is derived from polydopamine (PDA). The surface of the shell is modified with a vascular endothelial growth factor (VEGF) mimic peptide (QK peptide). TPQ was then embedded in porous methacrylate gelatin (GelMA) to form a TPQGel hydrogel. In the inflammatory environment, the TPQGel hydrogel can gradually release drugs through pH responsiveness, promoting M2 macrophage polarization, vascularization and bone regeneration in turn. In addition, reprogrammed M2 macrophages stimulate the generation of anti-inflammatory and pro-healing growth factors, which provide additional support for angiogenesis and bone regeneration. The TPQGel hydrogel can not only accurately fill irregular bone defects but also has excellent biocompatibility, making it highly suitable for the minimally invasive treatment of bone defects. Transcriptomic tests revealed that the TPQGel hydrogel achieved macrophage reprogramming by regulating the PI3K-AKT signalling pathway. Overall, the TPQGel hydrogel can be harnessed for safe and efficient therapeutics that accelerate the repair of bone defects.
Keywords: Bone defects; Bone regeneration; Core‒shell structure; Inflammatory microenvironment; M2 macrophage polarization; Vascularization.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The experimental protocol was reviewed and approved by the Animal Ethics Committee of Shanghai Public Health Clinical Center (approval number 2023-A032-01). Competing interests: The authors declare no competing interests.
Figures

References
-
- Wu M, Liu H, Zhu Y, Chen F, Chen Z, Guo L, Wu P, Li G, Zhang C, Wei R, et al. Mild Photothermal-Stimulation based on injectable and photocurable hydrogels orchestrates Immunomodulation and osteogenesis for High‐Performance bone regeneration. Small. 2023;19. 10.1002/smll.202300111. - PubMed
-
- Liu Q, Zhang S, Shi L, Shi J, Sun C, Wang J, Zhou W, Zhou H, Shan F, Wang H, et al. Osteogenic induction and Anti-Inflammatory effects of Calcium‐Chlorogenic acid nanoparticles remodel the osteoimmunology microenvironment for accelerating bone repair. Adv Healthc Mater. 2024;13. 10.1002/adhm.202401114. - PubMed
-
- Han S, Yang H, Ni X, Deng Y, Li Z, Xing X, Du M. Programmed release of vascular endothelial growth factor and exosome from injectable Chitosan nanofibrous microsphere-based PLGA-PEG-PLGA hydrogel for enhanced bone regeneration. Int J Biol Macromol. 2023;253. 10.1016/j.ijbiomac.2023.126721. - PubMed
-
- van der Heide D, Cidonio G, Stoddart MJ, D’Este M. 3D printing of inorganic-biopolymer composites for bone regeneration. Biofabrication. 2022;14. 10.1088/1758-5090/ac8cb2. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

