Medication-related perceptions of children and adolescents with severe asthma and moderate-to-severe atopic dermatitis: a non-interventional exploratory study
- PMID: 40197393
- PMCID: PMC11978019
- DOI: 10.1186/s13223-025-00961-8
Medication-related perceptions of children and adolescents with severe asthma and moderate-to-severe atopic dermatitis: a non-interventional exploratory study
Abstract
Background: Severe asthma and moderate-to-severe atopic dermatitis can significantly impact the lives of children and adolescents. However, real-world data on pediatric patients' perceptions of their medication are limited.
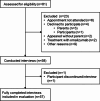
Methods: This non-interventional cross-sectional study at a university hospital explored patients' perceptions. We included patients aged between 6 and 17 with severe asthma and/or moderate-to-severe atopic dermatitis. For patients treated with dupilumab, a minimum dupilumab treatment duration of 16 weeks was required. We conducted one structured interview per patient, based on a questionnaire consisting of open questions and ratings on 6-point Likert scales (response scale range: "0: not at all" to "5: very strongly").
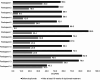
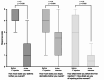
Results: The study included 57 participants (severe asthma: n = 31; moderate-to-severe atopic dermatitis: n = 21; both: n = 5) who reported a "rather moderate" burden of asthma (median: 2; Q25/Q75: 0.3/2.8) or atopic dermatitis (3; 1.5/3.5). They experienced their current medications as "rather helpful" (asthma: 4; 3/5; atopic dermatitis: 4; 3/5). Twelve of the participants (21%) reported refusing to take their medication because of reluctance, but all resumed treatment. All participants receiving dupilumab therapy (n = 16) reported an improvement in their disease within a maximum of 2.5 months after starting treatment. The median fear of injection decreased from 3 (0/5) before the first injection to 0.5 (0/1) at the time of the survey.
Conclusions: In this real-world, interview-based study, we found that pediatric patients perceived treatment as highly beneficial for asthma and atopic dermatitis. Furthermore, pediatric patients seemed to respond well to dupilumab therapy in terms of both disease improvement and less fear of injection.
Trial registration: DRKSID DRKS00028092.
Keywords: Antibodies, monoclonal; Asthma; Dermatitis, atopic; Drug therapy; Pediatrics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee at the Medical Faculty of Leipzig University (Reg. no: 050/22-ek). Written informed consent was obtained from all individual participants included in the study. Consent for publication: Not applicable. Competing interests: A.B. reports grants from UCB Pharma GmbH and honoraria for speaking engagements from Biogen GmbH, Desitin Arzneimittel GmbH, Eisai GmbH, GW Pharma GmbH, Jazz Pharmaceuticals GmbH, Neuraxpharm GmbH, Shire/Takeda GmbH, UCB Pharma GmbH, and ViroPharma GmbH. F.P. reports personal fees from Sanofi Aventis, Allergopharma, Stallergenes Greer, Vertex Pharmaceuticals, Takeda Pharma, and ALK-Abelló. T.L. reports honoraria for speaking engagements from Sanofi-Aventis, Novartis, and Aimmun. All reported grants, fees and honoraria are not related to the content of the manuscript. The other authors declare they have no conflicts of interest.
Figures
References
-
- Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. 2020;42:5–15. 10.1007/s00281-020-00785-1 - PubMed
-
- Ramírez-Marín HA, Silverberg JI. Differences between pediatric and adult atopic dermatitis. Pediatr Dermatol. 2022;39:345–53. 10.1111/pde.14971 - PubMed
-
- Silva N, Carona C, Crespo C, Canavarro MC. Quality of life in pediatric asthma patients and their parents: a meta-analysis on 20 years of research. Expert Rev Pharmacoecon Outcomes Res. 2015;15:499–519. 10.1586/14737167.2015.1008459 - PubMed
-
- Blome C, Radtke MA, Eissing L, Augustin M. Quality of life in patients with atopic dermatitis: disease burden, measurement, and treatment benefit. Am J Clin Dermatol. 2016;17:163–9. 10.1007/s40257-015-0171-3 - PubMed
LinkOut - more resources
Full Text Sources

