Early initiated noradrenaline versus fluid therapy for hypotension and shock in the emergency department (VASOSHOCK): a protocol for a pragmatic, multi-center, superiority, randomized controlled trial
- PMID: 40197397
- PMCID: PMC11978030
- DOI: 10.1186/s13049-025-01369-4
Early initiated noradrenaline versus fluid therapy for hypotension and shock in the emergency department (VASOSHOCK): a protocol for a pragmatic, multi-center, superiority, randomized controlled trial
Abstract
Background: Shock is a condition with high mortality even with early intervention and treatment. Usual care for shock and hypotension in the Emergency Department (ED) is intravenous fluid resuscitation which can lead to fluid overload and other complications. When fluid therapy fails or risk of complications are high, the next treatment step is the use of vasopressors for stabilisation. Noradrenaline therapy for hypotension and shock are commonly used in ED's outside Scandinavia, but the evidence on the optimal initiation time is sparse. The lack of noradrenaline therapy in Scandinavia provides a unique environment to investigate the possible implications of early initiation. The aim of this trial is to investigate whether the use of early initiated noradrenaline compared to ED fluid therapy can improve blood pressure goals and by that, reduce the need for ICU admittance.
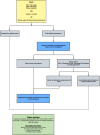
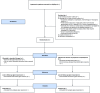
Methods: This protocol describes a pragmatic, multi-center, superiority randomized controlled trial, randomizing patients with hypotension to intervention or control. Eligible patients are ≥ 18-year-old who have received at least 500 ml intravenous fluids (including prehospital administration), and without suspected cardiogenic, haemorrhagic, anaphylactic, or neurogenic causes, or require direct ICU admittance due to non-hemodynamic severe organ failure. The intervention group receives noradrenaline initiated at 0.05 mcg/kg/min with a maximum of 0.15 mcg/kg/min through a peripheral venous catheter for up to 24 h. The control group receives usual care. Treatment is targeted for a systolic blood pressure ≥ 100 mmHg, a mean arterial pressure ≥ 65 mmHg or a clinician defined blood pressure target. We require a sample size of 320 patients to show a significant difference in proportion of patients achieving shock control within 90 min (primary endpoint). Key secondary outcomes include ICU free days alive within 30-days and 30-day all-cause mortality.
Discussion: Previous prospective randomized trials on early peripheral noradrenaline treatment for shock are sparse and are investigated in settings where noradrenaline use is already usual care. Since noradrenaline are not used as standard treatment for shock in Scandinavian EDs, this provides a unique opportunity not only to investigate the early initiation of noradrenaline for shock, but also comparing it directly to ED fluid only approach.
Trial registration: EU CT ID 2023-504584-16-00.
Clinicaltrials: gov NCT05931601. URL: https://classic.
Clinicaltrials: gov/ct2/show/NCT05931601.
Keywords: Emergency department; Emergency medicine; Fluid therapy; Hypotension; Noradrenaline; Norepinephrine; Resuscitation; Shock; Vasopressor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent for participation: The trial is approved by the Danish Medicines Agency and The Ethical Committee on Medical Research under European Medical Medicines Agency under EU CT ID 2023-504584-16-00. In addition, the trial is registered at clinicaltrials.gov under ID NCT05931601. Patients in the trial are included under the regulation for acute drug trials following the EU regulation no. 536/2014, Chapter V, Article 35 [34]. Inclusion of trial participants are therefore conducted without prior consent from the patient due to the severity and time-critical condition. The possibility to obtain informed consent is not possible due to the patient’s mental state in the early stages of their condition. After patients are included, the investigators will seek to obtain informed consent in accordance with national law and regulations in the individual participation country. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical