Activation of SIRT1 Reduces Renal Tubular Epithelial Cells Fibrosis in Hypoxia Through SIRT1-FoxO1-FoxO3-Autophagy Pathway
- PMID: 40197776
- PMCID: PMC12264462
- DOI: 10.1002/adbi.202400583
Activation of SIRT1 Reduces Renal Tubular Epithelial Cells Fibrosis in Hypoxia Through SIRT1-FoxO1-FoxO3-Autophagy Pathway
Abstract
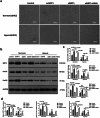
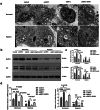
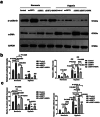
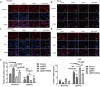
Heart failure-induced renal tubular epithelial cell fibrosis is an important pathological process that leads to chronic kidney disease. This study is to investigate the regulatory mechanism of over-expression or knock-down SIRT1 gene, alleviating hypoxia-induced HK2 cell fibrosis in heart failure. The focus is on the SIRT1-FoxO1-FoxO3-Autophagy pathway. In vitro experiments are performed by HK2cell line to simulate the normal oxygen state (Normoxia) and the hypoxia state (Hypoxia) caused by heart failure, SIRT1 gene over-expression by transfected vectors, knock-down and Rapamycin (RAPA)-induced cellular autophagy, and the cell models are divided into four subgroups, named control group, oeSIRT1, siSIRT1 and siSIRT1+RAPA. Western blotting (WB), real-time qPCR, immunofluorescence (IF), ELISA, and transmission electron microscopy are used to quantitatively or semi-quantitatively analyze the expression of FoxO1, FoxO3, SIRT1, Beclin1, LC-3, α-SMA, E- Cadherin, and collagen-I in cells or supernatants. It is demonstrated that activation of SIRT1 regulates the expression and activity of FoxO1 and FoxO3, thereby affecting autophagy. This modulation leads to a reduction in HK2 fibrosis markers (α-SMA and E-cadherin) and extracellular matrix deposition (collagen I), which ultimately attenuates renal tubular epithelial cell fibrosis. These findings provide new insights into potential therapeutic strategies for treating heart failure-induced renal tubular epithelial cell fibrosis by targeting the SIRT1-FoxO1-FoxO3-Autophagy pathway.
Keywords: SIRT1; autophagy; fibrosis; foxO1; heart failure; hypoxia.
© 2025 The Author(s). Advanced Biology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
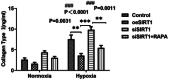
Similar articles
-
Fat mass and obesity-associated protein downregulation trigger the activation of the sirtuin 1/forkhead box O1 signaling pathway, drive glycolysis, and promote the progression of renal cell carcinoma.Cytojournal. 2025 May 9;22:51. doi: 10.25259/Cytojournal_33_2025. eCollection 2025. Cytojournal. 2025. PMID: 40539119 Free PMC article.
-
miR-210 Regulates Autophagy Through the AMPK/mTOR Signaling Pathway, Reduces Neuronal Cell Death and Inflammatory Responses, and Enhances Functional Recovery Following Cerebral Hemorrhage in Mice.Neurochem Res. 2025 Jun 5;50(3):180. doi: 10.1007/s11064-025-04434-7. Neurochem Res. 2025. PMID: 40471451 Free PMC article.
-
Txnip promotes autophagic apoptosis in diabetic cardiomyopathy by upregulating FoxO1 and its acetylation.Cell Signal. 2024 Dec;124:111469. doi: 10.1016/j.cellsig.2024.111469. Epub 2024 Oct 11. Cell Signal. 2024. PMID: 39396562
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Mitochondrial dysfunction and aging: multidimensional mechanisms and therapeutic strategies.Biogerontology. 2025 Jul 9;26(4):142. doi: 10.1007/s10522-025-10273-4. Biogerontology. 2025. PMID: 40634825 Free PMC article. Review.
References
-
- Minhas A. M. K., Ijaz S. H., Jamal S., Dani S. S., Khan M. S., Greene S. J., Fudim M., Warraich H. J., Shapiro M. D., Virani S. S., Nasir K., Khan S. U., Circ. Heart Fail 2022, 15, 008943. - PubMed
-
- Tolwani A., N. Engl. J. Med. 2012, 367, 2505. - PubMed
-
- Johansson I., Joseph P., Balasubramanian K., McMurray J. J. V., Lund L. H., Ezekowitz J. A., Kamath D., Alhabib K., Bayes‐Genis A., Budaj A., Dans A. L. L., Dzudie A., Probstfield J. L., Fox K. A. A., Karaye K. M., Makubi A., Fukakusa B., Teo K., Temizhan A., Wittlinger T., Maggioni A. P., Lanas F., Lopez‐Jaramillo P., Silva‐Cardoso J., Sliwa K., Dokainish H., Grinvalds A., McCready T., Yusuf S., Circulation 2021, 143, 2129. - PubMed
-
- Free D., https://www.mdpi.com/2075‐4418/14/15/1635, (accessed: July 31, 2024).
-
- Shanah L., Mir T., Uddin M. M., Hussain T., Parajuli T., Bhat Z., Int. J. Cardiol. 2023, 370, 244. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

