What Is Gestational Diabetes-Really?
- PMID: 40197830
- PMCID: PMC12185965
- DOI: 10.2337/dbi24-0041
What Is Gestational Diabetes-Really?
Abstract
Gestational diabetes mellitus (GDM) is one of the most common medical complications of pregnancy. It is generally defined as glucose intolerance with onset or first recognition during pregnancy. The pathogenesis of GDM has long been attributed to inadequate pancreatic β-cell compensation for the physiological insulin resistance of pregnancy. This defect is thought to resolve after pregnancy but become manifest in later life as an increased risk of diabetes. Examination of mechanisms underlying GDM does not support this commonly held picture. In this Perspective, we present evidence that, like diabetes outside of pregnancy, GDM has no single etiology. It results from multiple causes of a common physiological manifestation, inadequate β-cell function, which leads to a common clinical manifestation, elevated glucose levels. We provide evidence that GDM often represents detection of chronic and progressive β-cell dysfunction that is temporally but not mechanistically related to pregnancy. We provide detailed characterization of the β-cell defect in one high-risk group, Hispanic Americans. Finally, we address some of the clinical and research implications of these findings.
Article highlights: Gestational diabetes mellitus (GDM) is not one disease but many that share inadequate β-cell function as a common cause for elevated glucose levels. Inadequate β-cell function may result from factors that occur outside of pregnancy, such as autoimmunity, monogenic disorders, obesity, and insulin resistance. Pregnancy-specific causes may exist as well but remain to be defined. Detailed physiological studies in women with obesity reveal that inadequate β-cell function is likely a chronic condition that is detected by routine glucose screening in pregnancy and that worsens over time, leading to diabetes in later life. The authors' studies in Hispanic patients identify obesity and insulin resistance as important causes of β-cell dysfunction, providing a rationale for treating both to prevent diabetes after GDM. Additional work is needed to define the full breadth of underlying causes of GDM as the basis for precision management during and, especially, after pregnancy.
© 2025 by the American Diabetes Association.
Conflict of interest statement
Figures

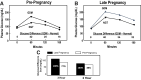



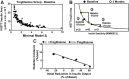

References
-
- Petersen JS, Dyrberg T, Damm P, Kühl C, Mølsted-Pedersen L, Buschard K. GAD65 autoantibodies in women with gestational or insulin dependent diabetes mellitus diagnosed during pregnancy. Diabetologia 1996;39:1329–1333 - PubMed
-
- Weng J, Ekelund M, Lehto M, et al. Screening for MODY mutations, GAD antibodies, and type 1 diabetes–associated HLA genotypes in women with gestational diabetes mellitus. Diabetes Care 2002;25:68–71 - PubMed
-
- Löbner K, Knopff A, Baumgarten A, et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes 2006;55:792–797 - PubMed
-
- Kousta E, Ellard S, Allen LI, et al. Glucokinase mutations in a phenotypically selected multiethnic group of women with a history of gestational diabetes. Diabet Med 2001;18:683–684 - PubMed
-
- Ellard S, Beards F, Allen LI, et al. A high prevalence of glucokinase mutations in gestational diabetic subjects selected by clinical criteria. Diabetologia 2000;43:250–253 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DK116858 R01DK134079 R01DK46334 R01DK46374 R01D/U.S. Department of Health and Human Services National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases
- UL1TR001855/U.S. Department of Health and Human Services National Institutes of Health National Center for Advancing Translational Sciences
- R01 DK046374/DK/NIDDK NIH HHS/United States
- Takeda Pharmaceuticals North America
- R01 DK061628/DK/NIDDK NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- Merck &Co
- UL1 TR001855/TR/NCATS NIH HHS/United States
- Parke-Davis Pharmaceutical Research
- R01 DK134079/DK/NIDDK NIH HHS/United States
- R01 DK116858/DK/NIDDK NIH HHS/United States
- M01RR00043/U.S. Department of Health and Human Services National Institutes of Health National Center for Research Resources
- 1-14-ACE-36 7-04-DCS-03 7-09-CT/American Diabetes Association
LinkOut - more resources
Full Text Sources

