Gene therapy for hemophilia B: results from the phase 1/2 101HEMB01/02 studies
- PMID: 40197980
- PMCID: PMC12197983
- DOI: 10.1182/bloodadvances.2024015184
Gene therapy for hemophilia B: results from the phase 1/2 101HEMB01/02 studies
Abstract
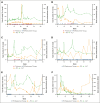
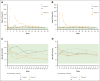
Hemophilia B is a rare, X-linked bleeding disorder that predominantly affects males and is caused by factor IX (FIX) gene variants, leading to spontaneous bleeding and impaired ability to clot after injury or surgeries. Standard of care is prophylaxis to increase FIX levels. DTX101 is a nonreplicating adeno-associated viral serotype rh10 gene transfer vector containing a codon-optimized wild-type human FIX coding sequence. The phase 1/2 open-label, single-arm, multicenter, dose-finding 101HEMB01 study examined the safety/efficacy of DTX101 in adult males with hemophilia B; the 101HEMB02 follow-up study assessed long-term outcomes. Participants received DTX101 as 1.6 × 1012 (cohort 1; n = 3) or 5.0 × 1012 genome copies/kg (cohort 2; n = 3) at baseline and were monitored through week 44 (cohort 2) or 52 (cohort 1) in 101HEMB01, and 4 additional years in 101HEMB02. The primary end point of 101HEMB01, peak plasma FIX level at week 6, showed median levels of 7.0 (range, 5.0-8.0) and 10.0 IU/dL (range, 6.0-16.0) in cohorts 1 and 2, respectively. Levels failed to reach the 20 IU/dL target criteria; all participants required adjunct FIX replacement therapy based on low FIX activity at intermediate time points. In 101HEMB01, 4 of 6 participants experienced treatment-related adverse events of elevated transaminase levels (n = 3) and fatigue (n = 1), and 1 experienced fatigue in 101HEMB02; none experienced related serious adverse events. Elevated transaminase levels were asymptomatic and resolved with steroids in all participants. The DTX101 program was halted for insufficient treatment response; however, from its completion, lessons can be learned regarding the design and execution of gene therapy clinical trials, including additional optimization of transgene sequence and immunosuppression protocols. The 101HEMB01 and 101HEMB02 studies were registered at www.ClinicalTrials.gov as #NCT02618915 and #NCT02971969, respectively.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.P. reports consultancy fees from Apcintex/Centessa, ASC Therapeutics, Bayer, BioMarin, CSL Behring, HEMA Biologics, Freeline, LFB, Novo Nordisk, Pfizer, Precision Biosciences, Regeneron/Intellia, Roche/Genentech, Sanofi, Takeda, Spark Therapeutics, and uniQure; research funding from Siemens and YewSavin, Inc.; and scientific advisory board fees from GeneVentiv and Equilibra Bioscience. A.R. reports research support to institution from BioMarin, Dimension Therapeutics, Janssen Momenta Pharmaceuticals, Takeda, and Tremeau Pharmaceuticals. S.S., J.A., J.C., and E.C. are employees/shareholders of Ultragenyx Pharmaceutical Inc. The remaining authors declare no competing financial interests.
The current affiliation for A.P. is Zenas BioPharma, Waltham, MA.
The current affiliation for J.A. is Vertex Pharmaceuticals, Boston, MA.
Figures


References
-
- Rallapalli PM, Kemball-Cook G, Tuddenham EG, Gomez K, Perkins SJ. An interactive mutation database for human coagulation factor IX provides novel insights into the phenotypes and genetics of hemophilia B. J Thromb Haemost. 2013;11(7):1329–1340. - PubMed
-
- Konkle BA, Huston H, Fletcher SN. Hemophilia B. In: GeneReviews. Adam MP, Mirzaa GM, Pagon RA, editors. University of Washington; Seattle: 1993-2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

