Gut microbiota restoration with oral pooled fecal microbiotherapy after intensive chemotherapy: the phase 1b CIMON trial
- PMID: 40197991
- PMCID: PMC12305571
- DOI: 10.1182/bloodadvances.2024015571
Gut microbiota restoration with oral pooled fecal microbiotherapy after intensive chemotherapy: the phase 1b CIMON trial
Abstract
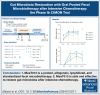
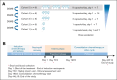
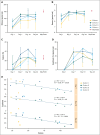
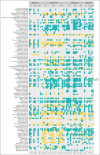
Intensive induction chemotherapy (IC) combined with broad-spectrum antibiotics for acute myeloid leukemia (AML) leads to gut microbiota dysbiosis, promoting pathological conditions and an increased incidence of complications, possibly limiting eligibility to allogeneic hematopoietic cell transplantation (alloHCT). The purpose of this dose-ranging phase 1 study (CIMON) was to evaluate the first-in-man use of MaaT033, a pooled, allogeneic, lyophilized, and standardized fecal microbiotherapeutic product, formulated as a delayed-release capsule for oral administration. Primary objectives of the study were to evaluate the maximum tolerable dose of MaaT033 in 21 patients with AML having undergone IC and antibiotics. Secondary objectives were to assess MaaT033 safety, its efficacy in restoring the patients' gut microbiome using shotgun sequencing to evaluate the recommended dose regimen, and patient compliance. MaaT033 was shown to be safe and effective for gut microbiota restoration in patients with AML receiving IC and antibiotics, with an excellent gut microbiota reconstruction based on diversity indices at the species level and restoration of microbial communities close to the composition of the drug product. The maximum tolerable dose of MaaT033 was not determined because the interim results suggested adequate efficacy as measured by engraftment at lower doses (3 capsules per day). Moreover, inflammatory markers (C-reactive protein, interleukin-6) decrease with treatment, whereas short-chain fatty acids increase over time. A randomized, placebo-controlled phase 2b trial, in recipients of alloHCT patients is ongoing. This trial was registered at www.clinicaltrials.gov as #NCT04150393.
© 2025 American Society of Hematology. Published by Elsevier Inc. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
Conflict-of-interest disclosure: F.M. reports honoraria from Bristol Myers Squibb (BMS), Therakos/Mallinckrodt, Sanofi, Priothera, Novartis, AstraZeneca, and Merck Sharp & Dohme, all outside the submitted work. S.T. reports honoraria from BMS, Gilead, Astellas, and Therakos/Mallinckrodt. T.C. reports clinical research support from Novartis, Alexion, Celgene/BMS, Amgen, Syros, Kartos, Arog, Takeda, Servier, Janssen, and Keros; consulting or advisory role with BMS/Celgene, AbbVie, Jazz Pharmaceuticals, Novartis, Agios, Servier, Astellas, Incyte, and BluePrint; and support for attending meetings and/or travel from Pfizer, Celgene/BMS, Novartis, AbbVie, Servier, and Gilead. M.L. reports honoraria from AstraZeneca, Alexion, BMS/Celgene, Pfizer, Gilead, Novartis, Kartos, Telios, GSK, Stemline, Incyte, Merck Sharp & Dohme, and Janssen. M. Meunier reports honoraria from BMS, Novartis, Alexion, Pfizer, and GSK, all outside the submitted work. E. Plantamura, B.L., and C.G. are employees of MaaT Pharma and have patent WO2020/016445 A1. J.J., E. Prestat, and A.S. are employees of MaaT Pharma. J.D. is a cofounder and a member of the advisory board of MaaT Pharma; reports consulting fees from MaaT Pharma; reports support for attending meetings and/or travel from MaaT Pharma; and reports shares from MaaT Pharma and patents WO2016/170285 A1, WO2016/170290 A1, and WO2017/103550 A1. C.R. declares a consulting or advisory role with AbbVie, Amgen, Astellas, BMS, Boehringer, Jazz Pharmaceuticals, and Servier; reports receiving research funding from AbbVie, Amgen, Astellas, BMS, Iqvia, Jazz Pharmaceuticals, and MaaT Pharma; and reports support for attending meetings and/or travel from AbbVie, Novartis, and Servier. M. Mohty reports grants, lecture honoraria, and research support from Adaptive Biotechnologies, Amgen, Astellas, BMS/Celgene, GlaxoSmithKline, Janssen, Jazz Pharmaceuticals, Novartis, Pfizer, Takeda, and Sanofi, all outside the scope of this work. The remaining authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

